Review Article
Gene Silencing and its Applications in Plants
Norah M. Al Aboud*
Department of Biology, Umm Al-Qura University,Saudi Arabia
*Corresponding author: Norah M. Al Aboud, Department of Biology, Umm Al-Qura University, Saudi Arabia
Copyright: © Al Aboud NM. 2022. This is an open access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 11/11/2022; Accepted: 12/12/2022; Published: 16/12/2022
Abstract
Gene silencing (GS) is considered a promising tool for studying gene functions, improving various crop traits as well as providing resistance to pathogens.
Silencing may be done at the transcript or at the post-transcript level. Gene silencing can be done using either RNAi or CRISPR. RNAi silences genes by
generating knockdowns at the mRNA level, while CRISPR generates knockouts at the DNA level. Genetic silencing played an important role in protecting
plants from pathogens, silencing the synthesis pathways of many compounds in plants such as nicotine, caffeine and gluten, as well as improving the quality
of fruits and prolonging their shelf life, but silencing by traditional methods requires genetic modification of the plant and this takes a long time in addition
to these, genetically modified plants has faced great rejection from most societies. Induction of gene silencing by external spraying of dsRNA molecules
complementary to the pathogen’s gene on plant is one of the modern, fast, inexpensive, and environmentally friendly methods that do not require genetic
modification of plant and will enhance plant resistance against many pathogens. This application is recognized as spray-induced gene silencing (SIGS).
Keywords
Gene silencing;RNAi; miRNA; siRNA; HIGS; SIGS
Introduction
Gene silencing is defined as an epigenetic modification of gene
expression leading to inactivation of previously active genes. Epigenetic
modification does not alter the DNA sequence and, although it
is heritable, variable frequencies of reversions to expression are
observed. Gene silencing is used in the course of normal development
and differentiation to repress genes whose products are not required
in specific cell types or tissues. This may apply to individual genes
or larger chromosome regions [1]. Mechanisms responsible for
repression of genes involve changes in levels of DNA methylation,
alterations in covalent modifications of histone proteins, chromatin
compaction, or destabilization of mRNA. Particular patterns of
modifications of chromatin proteins and DNA template make genes
inaccessible to the transcription machinery. mRNA destabilization
and repression of mRNA translation are often mediated by small RNA
regulators such as microRNAs (miRNAs) and small interfering RNAs
(siRNAs) [2,3].
Gene silencing can act at the transcriptional or post transcriptional
level; the two phenomena being referred to as transcriptional gene
silencing (TGS) and posttranscriptional gene silencing (PTGS). Genes
affected by TGS are not transcribed at all, or transcripts are produced
at very low levels. TGS has been observed in fungi, plants, and
animals. In PTGS, also referred to as cosuppression in plants, quelling,
or RNA interference (RNAi), the affected gene is transcriptionally
active but its transcripts undergo rapid degradation, resulting in the
absence of translatable mRNA [1-4].The discovery of mechanisms
that suppress gene activity in plants has extended the horizon for
research on control of gene expression (Mansoor et al., 2006) [5].
Gene silencing has also been used in food quality modification such
as the reduction of caffeine levels in coffee beans [6], and to increase
the nutritional value of corn protein and tomatoes [7,8]. Research on
forest tree yield and quality has included the study of gene silencing
related to lignin synthesis. On the other hand, research on fruit crops
has targeted applications of gene silencing on viral and bacterial
resistance, and physiological aspects such as self-fertility. The study
of plant gene function by affecting gene expression through silencing
techniques (PTGS / RNAi and VIGS) has also been present in recent
lines of investigation. This review reports and discusses the main
molecular mechanisms involved in plant gene silencing, compares the
mechanisms and experimental workfl ow and the applications of this
technology in plant improvement.
Literature Review:
The world population is estimated to reach 11.2 billion by 2100
and the global food supply must be continuously improved to meet
such population growth [9]. Minimizing the crop losses due to pests
and diseases and alter the gene expression for better quality traits are
crucial for future sustainability of global crop production. The global
agricultural direct yield loss is estimated between 20 and 40%, which
are mainly contributed by pathogens, animals, and weeds [10-13].The initial idea of gene silencing was discovered when an effort
to overexpress chalcone synthase (CHS) in pigmented petunia
petals by introducing a chimaeric petunia CHS gene has blocked the
biosynthesis of anthocyanin, resulting in totally white fl owers and/
or patterned fl owers with white or pale non-clonal sectors on a wildtype
pigmented background [14]. However, the mechanism causing
suppression of the target gene was unknown. The RNA gene silencing
mechanism was later discovered by injecting double-stranded RNA
(dsRNA) into the worm Caenorhabditis elegans which triggered the
silencing of genes with identical sequences to that of the dsRNA [15].
Since the discovery of RNA-mediated gene silencing mechanism,
this approach has been employed for elucidation of gene function
in plant [16]or to alter the gene expression for better quality traits,
such as development of seedless fruits [17], enhancement of shelf
life [18], development of male sterility and fertility[19] , nutritional
improvement, allergen and toxin elimination [20,21], and plant
protection.
Methods of Gene Silencing
RNA Interference (RNAi): According to the present model, the
RNA interference pathway starts with the presence of dsRNA in the
cytoplasm that vary in length and origin)[22-24] (Figure 1). This
particular molecule is recognized by the Dicer enzyme, a member
of the RNase III family of nucleases that specifically cleave double stranded
RNAs This enzyme cleaves the dsRNA into shorter RNA
duplexes of 21 to 28 nucleotides, which have 5’ phosphate and
2-nucleotide 3’ overhangs [23-25]. These short RNA duplexes are
known as short interfering RNA (siRNA) [26].Several organisms
contain more than one Dicer gene, with each Dicer preferentially
processing dsRNAs that come from specific source [27] Figure 1.
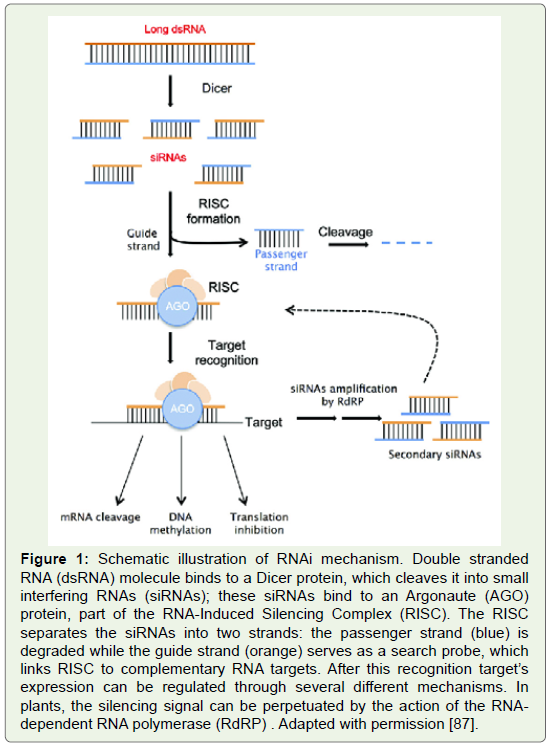
Figure 1: Schematic illustration of RNAi mechanism. Double stranded
RNA (dsRNA) molecule binds to a Dicer protein, which cleaves it into small
interfering RNAs (siRNAs); these siRNAs bind to an Argonaute (AGO)
protein, part of the RNA-Induced Silencing Complex (RISC). The RISC
separates the siRNAs into two strands: the passenger strand (blue) is
degraded while the guide strand (orange) serves as a search probe, which
links RISC to complementary RNA targets. After this recognition target’s
expression can be regulated through several diff erent mechanisms. In
plants, the silencing signal can be perpetuated by the action of the RNA dependent
RNA polymerase (RdRP) . Adapted with permission [87].
After Dicer processes the dsRNA, the siRNAs are subsequently
rearranged into the RNA-induced silencing complex (RISC)
[28,29]. The characterization of RISC includes the presence of an
Argonaute protein family member and a guide strand (antisense to
the target RNA) of a small RNA. The RISC complex is responsible
for the targeting and cleavage of sequence specific mRNA within
the cell. RISC acts by cleaving the target mRNA in the middle of the
complementary region, ten nucleotides upstream of the nucleotide
paired with the 5’ end of the guide siRNA [30]. At least one protein
from the Argonaute family, present in the RISC complex, probably
acts as endonuclease, cleaving the target mRNAs (often referred to
as the Slicer function) [31,32]. This cleavage leads to silencing of
the target mRNA by preventing read-through of the message by the
translational machinery, resulting in mRNA destruction.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR-Cas9): % of the sequenced bacterial genomes and
90% of the archaea [33], and has been identified to protect these
microbes from the future invasions by bacteriophages [34]. After
many years, the essential components of this system, including the
guide RNA (gRNA) which is essential to direct the Cas9 protein (an
important enzyme for induction of DNA double-strand break) to the
targeted region on the genome, have been identified. The CRISPRCas
9 system is now broadly applied to eukaryotes for editing genes,
including creating knock-in, knock-out, and also to correct the
mutated genes in the genome [35]. After the CRISPR-Cas9 system
induces a double strand break (DSB) at the targeted site (Figure 2),
the endogenous cellular repair mechanisms will be activated, and
can naturally attempt to repair and rejoin the broken DNA strands
through either of two mechanisms: (i) non-homologous end joining
(NHEJ) or (ii) homology-directed repair (HDR)[36,37]. Through
NHEJ, insertions and deletions (called indel mutations) of a small
number of the nucleotides is possible, and this might trigger a frameshift
mutation which can lead to the “loss-of-function” of protein coding
genes via the disruption of open reading frame (ORF) [38].
NHEJ-induced mutations can lead to silencing of a gene [39]-whereas,
using HDR, a large portion of the gene (2–10 kb) can be deleted and,
simultaneously, an incorporation of the exogenous DNA at the target
region of the genome is possible.
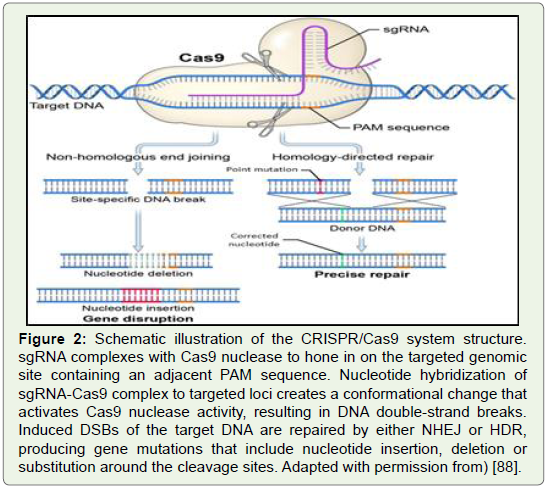
Figure 2: Schematic illustration of the CRISPR/Cas9 system structure.
sgRNA complexes with Cas9 nuclease to hone in on the targeted genomic
site containing an adjacent PAM sequence. Nucleotide hybridization of
sgRNA-Cas9 complex to targeted loci creates a conformational change that
activates Cas9 nuclease activity, resulting in DNA double-strand breaks.
Induced DSBs of the target DNA are repaired by either NHEJ or HDR,
producing gene mutations that include nucleotide insertion, deletion or
substitution around the cleavage sites. Adapted with permission from) [88].
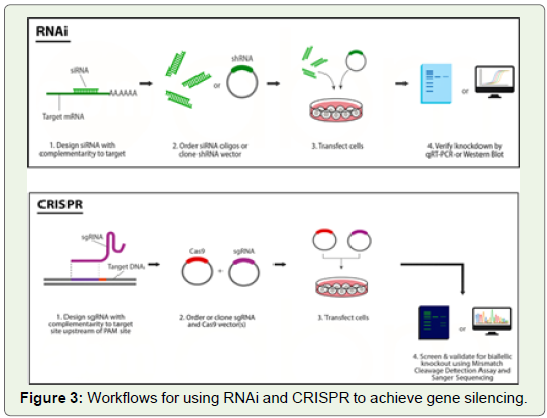
Experimental Workflow in RNAi and CRISPR:
Design of siRNAs easier than design of sgRNAs for CRISPR.
In order to design siRNAs, one only needs the sequence of the
corresponding mRNA transcript. While design for CRISPR requires
knowledge of the genomic DNA sequence, and CRISPR also depends
on the presence of a PAM sequence in the gene of interest. Depending
on the type of Cas9 used, the PAM sequence may be very common
within the genome (i.e. spCas9’s 5’-NGG-3`), or not as common
(i.e. saCas9’s 5`-NNGRRT-3`). In almost all cases a 5`-NGG-3` PAM
sequence will be present within the gene of interest.RNAi has the simplest experimental set up and siRNA treatment
can cause significant gene repression in only 24 hours [40]. Only one
transgene needs to be delivered into the cell. It can be prepared as
a ~20 bp double-stranded siRNA, or a ~80 bp shRNA cloned into
a vector. In comparison, CRISPR rely on exogenous nucleases that
must be delivered into the cell. This limits their effectiveness for use
with viral expression systems such as AAV (Adeno-Associated Virus),
which have limited packaging capacity.
No matter the method used, gene silencing must be verified before
conclusions can be made. The process for verifying gene silencing
varies depending on the technique used. When using RNAi, it’s best to
use two validation methods: one measuring mRNA levels (such as qRTPCR),
and another measuring protein levels (such as Western blot). A
decrease in mRNA levels seen without a corresponding decrease in
protein levels indicates that protein turnover may be slow. A decrease
in protein levels without a corresponding decrease in mRNA levels
indicates that the siRNA may be exerting its effects via translational
inhibition instead of mRNA degradation. CRISPR gene silencing
can be verified with methods that target the DNA. Initial screening
is usually performed using the Mismatch Cleavage Detection Assay
(a.k.a. Surveyor or T7E1), or by using Sanger sequencing (Figure 3).
Applications of Gene Silencing in Plants
Gene silencing was first used to develop plant varieties resistant to viruses. Engineered antiviral strategies in plants mimic natural RNA
silencing mechanisms. This was first demonstrated when scientists
developed Potato virus Y- resistant plants expressing RNA transcripts
of a viral proteinase [41]. Immunity has since been shown to other
viruses such as the Cucumber and Tobacco Mosaic Virus, Tomato
Spotted Wilt Virus, Bean Golden Mosaic Virus, Banana Bract Mosaic
Virus, and Rice Tungro Bacilliform Virus among many others.
In addition, plants can also be modified to produce dsRNAs that
silence essential genes in insect pests and parasitic nematodes. This
approach was used to develop root-knot nematode, corn rootworm
and cotton bollworm resistant varieties.
A spectacular example using gene silencing is the rescue of the
Hawaiian papaya industry by conferring resistance to papaya ringspot
virus (PRSV)[42]. Another notable achievement is the bioengineered
resistance of “NewLeaf Plus” potatoes to Potato leafroll virus, released
by Monsanto [43]. In Australia, Peter Waterhouse and his CSIRO
group pioneered the use of RNAi technology to develop varieties of
barley that are resistant to barley yellow dwarf virus(BYDV) [44].
Kusaba and colleagues applied RNAi to reduce the level of glutenin
in rice and produced a LGC-1 (low glutenin content 1) rice variety.
This low-protein rice is useful for patients with kidney disease whose
protein intake is restricted. The trait was stable and was transmitted
for a number of generations[45].
During chemical pulping of wood, one of the most expensive
and environmentally hazardous processes is to separate lignin from
cellulose and hemicellulose[46]. The production of plant material
with lower contents of lignin would mean a significant reduction
of cost and pollution to the paper industry. One of the approaches
to obtain reduced lignin forest trees has been the down regulation
of lignin biosynthesis pathways [47]. The main genes involved with
genetic transformation targeting lignin reduction are 4-coumarate:
coenzyme A ligase (Pt4CL1) cynnamyl alcohol deshydrogenase (CAD
- the final enzyme in the biosynthesis of lignin monomers) [48]and
caffeate/5-hydroxyferulate O-methyltransferase (COMT - enzyme
involved in syringyl lignin synthesis) [49].The downregulation of the
Pt4CL1 gene in PopulustremuloidesMichx., produced trees with a 45% reduction of the lignin content compensated by a 15% increase
in the cellulose content. In the transgenic lines obtained plant growth
was substantially enhanced, and structural integrity maintained
both at the cellular and whole-plant levelIn some woody plants, selfincompatibility
stands as a major problem in fruit set and breeding
programs[50], reported the production of transgenic apple trees able
to self-pollinate and develop fruit. This break through was achieved
by silencing of the S-gene responsible for self-incompatibility. The
self-compatible transgenic plants lacked the pistil S-RNase protein,
which is the product of the S-gene.
Fruit quality has also been addressed by silencing experiments.
Several characteristics are involved in fruit quality. Transgenic apple
fruits silencing key enzymes involved in autocatalytic ethylene
production were significantly firmer and displayed an increased shelf life
[51].
Asparagine plays an apparently important role in the assimilation
and storage of nitrogen [52], and is particularly abundant in the
products of wheat (Triticum aestivum) [53], coffee and potato [54,55].
On heat processing, the amide amino acid reacts with reducing sugars
to produce acrylamide [56].In humans, oral intake levels believed
to be without an appreciable risk of deleterious effects are currently
estimated to be 3.0 μg acrylamide/day (http://www.epa.gov/iris). This
level of dietary intake is exceeded in small subsets of the population,
particularly in young children and adolescents [57]. The Joint Food
and Agriculture Organization/World Health Organization (FAO/
WHO) Expert Committee on Food Additives and Contaminants
has therefore recommended reducing the acrylamide content of
processed starchy foods.
The faster route to decrease the acrylamide potential of food
crops was established through gene silencing. Simultaneous silencing
of two tuber-expressed genes in starch degradation, which encode
water dikinase R1 and amyloplast-targeted phosphorylase-L, led to a
decrease in the accumulation of glucose and fructose by approximately
twofold [58]. These modified tubers correlated with an approximately
two- to threefold decrease in acrylamide levels.
Among 12,000 alkaloids which are produced in plants, caffeine
(1,3,7-trimethylxanthine) is one of the best known. The demand
for decaffeinated coffee is growing globally, because of the possible
adverse health effects of caffeinated coffee. Caffeine can trigger
palpitations and increase blood pressure in sensitive individuals.
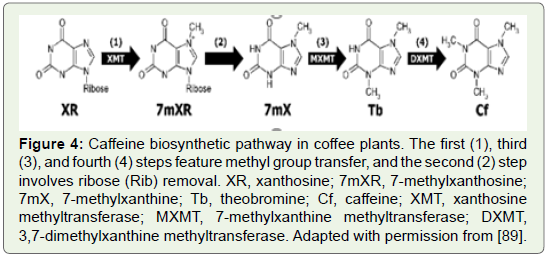
In coffee plants, caffeine is synthesized from xanthosine through
three successive methylation and ribose removal steps (Figure 4).
Ogita and his colleagues isolated all key genes for making caffeine
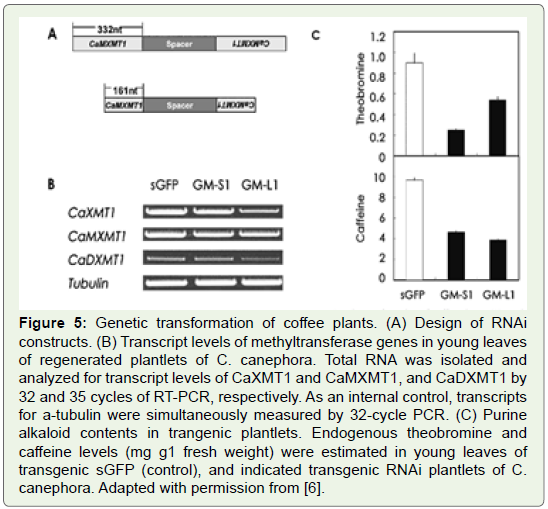
in coffee plants (Figure 5) and they were able to reduce decrease
the caffeine content in coffee plants by using an RNAi for MXMT,
yielding a 70% suppression of the caffeine level in leaves of transgenic
coffee plants (Figure 6).
Figure 4: Caffeine biosynthetic pathway in coffee plants. The first (1), third
(3), and fourth (4) steps feature methyl group transfer, and the second (2) step
involves ribose (Rib) removal. XR, xanthosine; 7mXR, 7-methylxanthosine;
7mX, 7-methylxanthine; Tb, theobromine; Cf, caffeine; XMT, xanthosine
methyltransferase; MXMT, 7-methylxanthine methyltransferase; DXMT,
3,7-dimethylxanthine methyltransferase. Adapted with permission from [89].
Figure 5: Genetic transformation of coffee plants. (A) Design of RNAi
constructs. (B) Transcript levels of methyltransferase genes in young leaves
of regenerated plantlets of C. canephora. Total RNA was isolated and
analyzed for transcript levels of CaXMT1 and CaMXMT1, and CaDXMT1 by
32 and 35 cycles of RT-PCR, respectively. As an internal control, transcripts
for a-tubulin were simultaneously measured by 32-cycle PCR. (C) Purine
alkaloid contents in trangenic plantlets. Endogenous theobromine and
caff eine levels (mg g1 fresh weight) were estimated in young leaves of
transgenic sGFP (control), and indicated transgenic RNAi plantlets of C.
canephora. Adapted with permission from [6].
Figure 6: GM decaff einated coff ee plants growing in a greenhouse. (A)
4-year old transgenic coff ee trees. Samples are RNAi (right side) and wild
type (left side). (B) Flowering of RNAi transgenic coffee plants. Adapted with
permission from (Ogita et al., 2005)
Worldwide, approximately 1.1 billion people are smokers
and more than 7 million people die from the negative effects of
smoking every year (WHO report, 2017). One of the main natural
ingredients causing dependence on tobacco is nicotine. Tobacco
with a lowered nicotine content could help people to overcome their nicotine addiction. Nicotine free (or nicotine reduced) cigarettes
may contribute to reduce the number of smokers and nicotine
consumption, thus reducing the risk of death from tobacco use. The
knockdown of the three most highly expressed BBL genes (BBLa–
BBLc) by RNAi or the knockout with EMS induced mutations
resulted in a reduction of the nicotine content without increasing
the content of other alkaloids [59,60].Recently, the BBL gene family
in tobacco was expanded by the identification of BBLd.2 and BBLe,
leading to six known isoforms [61]. Thus, the simultaneous knockout of these BBL genes is a promising approach to generate a nicotine free
tobacco plant.
Host-induced gene silencing (HIGS) is one of the methods that
have been used to enhance resistance against pathogens, by expressing
dsRNAs that target essential pathogen genes in host plant species
leading to disease resistance. In recent study, the HIGS approach was
successfully applied in maize and soybean to control the polyphagous
mirid bug, Apolyguslucorum (Liu et al., 2019). The selection of target
was based on a previous work of injection-based RNAi of seven
candidate genes in A. lucorum. The AlucV-ATPase-E gene was
selected as A. lucorum fed with dsRNA corresponding to this gene
has produced mortality rates of 46.01–82.32% at day 7 after injection.
Based on the above finding, the populations of A. lucorum were
significantly decreased after feeding on the transgenic maize and
soybean expressing the dsRNA targeting AlucVATPase-E gene.
In a recent study camerlengo and his colleagues used a multiplex
genome editing strategy to silent simultaneously two ATI genes (CM3
and CM16) in durum wheat, both indicated as major allergens in
bread and durum wheat [61-63], and likely to be involved in Non-
Coeliac Wheat Sensitivity (NCWS). This edited plants have potential
to be grown as safer durum wheat lines for individuals predisposed to
bakers’ asthma, food allergies, and NCWS[64].The study confirmed
that the multiplex genome editing system is an effective strategy to
suppress simultaneously more than one gene. A similar strategy has
been used to target two or more genes in wheat, rice and maize [65-67].
In another paper [30], the same ATI genes, plus the 0.28 gene,
were silenced in the bread wheat cultivar Bobwhite by using RNAi.
Different parameters related to yield resulted not affected, although
one related to dough quality was strongly affected due to the lower
expression of high molecular weight glutenin subunits, as an
unpredictable effect likely due to RNAi procedure.
Mechanisms of Higs and Sigs:
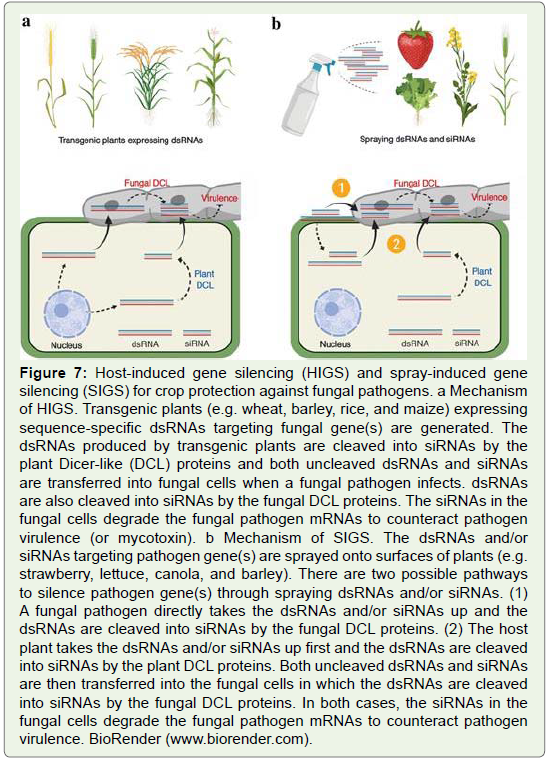
Host-induced gene silencing (HIGS) is an RNAi-based technique,
expressing sequence-specific dsRNAs in the host plant to silence target
genes of plant pathogens. A dsRNA or a hairpin-structured dsRNA
construct targeting a specific pathogen gene is transformed into
the host plant. The transgenic plant produces dsRNAs and siRNAs,
which find their entry into the plant pathogens during host pathogen
interactions (Figure 7a). The siRNAs degrade the pathogen mRNAs to
protect the host plant against the pathogen[]69-71.
Figure 7: Host-induced gene silencing (HIGS) and spray-induced gene
silencing (SIGS) for crop protection against fungal pathogens. a Mechanism
of HIGS. Transgenic plants (e.g. wheat, barley, rice, and maize) expressing
sequence-specific dsRNAs targeting fungal gene(s) are generated. The
dsRNAs produced by transgenic plants are cleaved into siRNAs by the
plant Dicer-like (DCL) proteins and both uncleaved dsRNAs and siRNAs
are transferred into fungal cells when a fungal pathogen infects. dsRNAs
are also cleaved into siRNAs by the fungal DCL proteins. The siRNAs in the
fungal cells degrade the fungal pathogen mRNAs to counteract pathogen
virulence (or mycotoxin). b Mechanism of SIGS. The dsRNAs and/or
siRNAs targeting pathogen gene(s) are sprayed onto surfaces of plants (e.g.
strawberry, lettuce, canola, and barley). There are two possible pathways
to silence pathogen gene(s) through spraying dsRNAs and/or siRNAs. (1)
A fungal pathogen directly takes the dsRNAs and/or siRNAs up and the
dsRNAs are cleaved into siRNAs by the fungal DCL proteins. (2) The host
plant takes the dsRNAs and/or siRNAs up first and the dsRNAs are cleaved
into siRNAs by the plant DCL proteins. Both uncleaved dsRNAs and siRNAs
are then transferred into the fungal cells in which the dsRNAs are cleaved
into siRNAs by the fungal DCL proteins. In both cases, the siRNAs in the
fungal cells degrade the fungal pathogen mRNAs to counteract pathogen
virulence. BioRender (www.biorender.com).
Spray-induced gene silencing (SIGS) is a novel non-transformative
strategy for plant protection. The dsRNA targeting a pathogen gene is
sprayed onto plant surfaces. The fungal pathogen directly takes the
dsRNAs up and induces the fungal RNAi machinery, and/or the host
plant takes dsRNAs up first, induces the plant RNAi machinery, and
then dsRNAs or siRNAs are transferred into fungal cells and induce
the fungal RNAi machinery (Figure 7b). Thus, this approach silences
pathogen’s gene without introducing heritable modifications into the
plant genome [72-74].
Applications of Gene Silencing in Plant via Non-Transgenic Approach:
Traditional application of RNA-mediated gene silencing to control various pests and diseases or improve crop features is through
transgenic approach, whereas the dsRNA/hpRNA construct must be
prepared and the transgenic plants need to be generated.However,
generation of these resistant transgenic plants may cause substantial
delay as it is highly dependent on the transformability and genetic
stability of the target crop plant species. Furthermore, limited
acceptance of the genetically modified organism (GMO) by consumer
will make the application less favourable. Recently, a number of studies
showed that the RNA gene silencingcould be induced by just spraying
dsRNA corresponding to the pathogen’s gene on plant. For instance,
topical applications of dsRNAs or siRNAs that target genes involved
in the ergosterol biosynthesis in Fusariumgraminearum (CYP51A,
CYP51B, and CYP51C), suppressed the fungal growth in barley [75].
The study found that the dsRNA was delivered to the distal parts of
detached leaves via the plant vascular system and processed by the
fungal DICER-LIKE1 (FgDCL-1) into siRNAs after being taken up
by the pathogen.Similarly, spraying wheat plants with the dsRNA
targeting myosine 5 gene of F. asiaticumreduced fungal virulence
[76]. In Brassica napus, exogenous applications of dsRNAs targeting
various genes of B. cinerea also decreased the gray mold disease
severity [77]. The dsRNAs or siRNAs targeting the B. cinereals iRNA
biosynthesis-related genes, such as Dicer-like 1 and 2 (DCL1 and
DCL2), significantly reduced the gray mold diseases in various fruits
and vegetables[78].This application is recognized as spray-induced
gene silencing (SIGS).The dsRNA molecules applied exogenously to the leaves showed a
fast-systemic spread from the treated (local) to non-treated (systemic)
leaves and was present for up to 9 days in local leaves and 6 days in
systemic leaves post-application. This approach offers another simple
and environmentally safe way for application of dsRNA in control of
pathogen virus. The possible workflow of the SIGS application was
summarized in (Figure 8) by giving an example to control the insect
pest.
However, there are a number of limitations in the application of
dsRNA which could disfavour its commercial potential. One of the
major limitations of the application of SIGS is the instability of naked
dsRNA sprayed on plants. The naked dsRNA is easily degraded with
the presence of soil or water. Several approaches, such as loading
the dsRNA into a layered double hydroxide (LDH) clay nanosheet,
namely the ‘Bioclay’ technology or guanidine-containing polymers
or encapsulation of dsRNA in liposome complexes, were found to
prolong the dsRNA shelf life under field condition [79-81]. A recent
study demonstrated that the dsRNA loaded in BioClay was not easily
washed of, showed sustained release under ambient conditions, and
could be detected on sprayed leaves even 30 days after application
[82]. In addition, the BioClay will provide RNAi-based systemic
protection to newly emerge unsprayed leaves as the SIGS approach
is effective on local and distal parts of the target plants[83,84].
The guanidine-containing polymers could protect dsRNA against
nucleolytic degradation especially under the high pH environments.
The dsRNA production cost is another concern. However,
production of the dsRNA using the in vitro or in vivo systems could
also contribute to the production cost saving [85-87]. Production of
dsRNA via in vivo expression in bacteria is preferable for the largescale
production [88-90]. An Apse RNA Containers™ (ARCs) was
developed recently by using the bacterial system for the biosynthesis
of dsRNA and capsid protein [91-95]. The bacteria co-transformed
with plasmids encoding the target dsRNA and capsid protein will
produce both components concurrently in the bacteria and the
protein subunits will be self-assembled around the dsRNA[96-101].
The encapsulated dsRNAs can be purified from the bacteria and
ready for field application. This technology will accelerate the mass
production of the dsRNA with longer stability, without involving high
production cost and complex procedures[102-107].