Research Article
Micropropagation and Conservation of a Wild Species of Solanum through Organ Cultures
Salahuddin K1*, Singh CK2, Md. Nizamuddin A2 and Naseem M2
1Department of Botany, L.N. Mithila University, India
2University Department of Botany, B.R.A Bihar University, India
*Corresponding author: Salahuddin K, Department of Botany, M.R.M College, L.N. Mithila University, Darbhanga-846004, Bihar, India, Phone: 9668816209, E-mail: salahuddin212@gmail.com
Copyright: © Salahuddin K, et al. 2020. This is an open access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 10/03/2020; Accepted: 02/04/2020; Published: 10/04/2020
Abstract
Micropropagation technique has emerged as the best tool for mass propagation of desired species. Solanum torvum Swartz, a wild species of crop
plants is in high demand for medicinal as well as breeding point of view. The existence of these wild plants is in danger due to induction of new cultivars and
other environmental hazards. In this background, there is urgent need for germplasm conservation and germplasm improvement besides mass propagation.
Keeping these objectives into consideration, tissue culture studies of this plant were being undertaken to develop protocol for in vitro mass propagation
and callogenesis. Regeneration of shoots and callus was obtained using sterilized segments of node (8-10 mm), internode (10-15 mm) and shoot-tip (8-10
mm) of Solanum torvum (about 2 years old). These explants were cultured on MS medium containing 0.8% agar, 3% sucrose and different combinations
and concentrations of NAA/2,4-D and Kinetin (Kn) to obtain regenerates / plantlets and callus differentiation. Techniques were used for shoot regeneration
directly from node and shoot-tip explants as well as from callus. Shoot regeneration was best achieved on 3mgl-1Kn in nodal and shoot-tip cultures. Callus
mediated shoot regeneration was promising in the culture and was obtained on 2 mgl-1 NAA and 4 mgl-1Kn on sub-culture. 2,4-D alone or 2,4-D + Kn resulted
in callus differentiation from explants. Callus in general was white/greenish-white, compact, hydrated and crystalline in appearance. Callus was maintained
for about 2 years on 1 mgl-1 NAA and 1 mgl-1 Kn on regular sub-culture after 25 days. Callus turned brown on higher concentration (10mgl-1) of auxin and Kn
on sub-culture. Rooting of microshoots (about 5 cm) was obtained on RM (½MS Salts) containing 1mgl-1 NAA and 2 mgl-1 IBA. Plantlets were successfully
transferred to soil and they survived well in nature. Explants taken during December to May were most regenerative. Plantlets obtained through in vitro were
morphologically identical to parent plants. Nodal explants were superior to other explants (internode, shoot-tip) with respect to shoot regeneration, where as
internodal explants was superior for callogenesis.
Keywords
Callus; Explant; Germplasm; In vitro; Regeneration
Introduction
The primitive cultivars and wild relatives of crop plants constitute
a pool of genetic diversity which is invaluable for future breeding
programme. However, the existence of these plants is in danger
due to induction of new cultivars and other environmental hazards.
Germplasm includes plant parts which are used for maintenance,
conservation and propagation of any biotype; it also acts as genetic
pool.
Tissue culture is a method of in vitro culture of cell, tissue and
organ in a sterile culture medium [1]. This technique can be referred
to as “Botanical laser” and its numerous uses are yet to be explored
and fully understood. The tools of plant tissue culture are being
applied to a wide range of biotechnology ventures and in particular
to the clonal propagation and genetic up gradation of crop and
medicinal plants [2]. In recent years, tissue culture techniques have
become useful tools in the hands of plant scientists of all disciplines
because these techniques are more handy, less time consuming and
less labour involving over the conventional methods of breeding and
propagation.
solanum torvum, (Fam: Solanaceae) commonly known as Devil’s
fig is a bushy perennial wild plant measuring 150-300 cm in height and usually growing in tropical and subtropical areas throughout the
world as a weed of disturbed areas. It is found growing in pastures,
road sides and wastelands but not significantly in cultivated land. It
prefers moist and fertile soil and also tolerates drought and saline
soils.
Fruits are eaten as vegetable and used as ingredient of pickles,
it is said to be good for enlargement of the spleen. Fruits contain a
number of potentially pharmacologically active chemicals including
sapogenin, steroid, sterolin, chlorogenin and solasonine. The aqueous
extracts of turkey berry (Solanum. torvum) were found lethal to mice
or depressed the erythrocytes, leukocytes and platelets in their blood.
Extracts of the plant are reported to be useful in the treatment of
hyperactivity, colds and cough, pimples, skin diseases and leprosy [3].
This plant is also used medicinally for the treatment of epilepsy [4].
Conservation of germplasm of this wild crop is highly needed
for developing perennial brinjal variety, a common vegetable
for millions of people of the world and its medicinal uses are also
required to be investigated in right perspectives. In this background,
it is necessary to multiply this plant through in vitro methods. Calli
and regenerates obtained through in vitro methods can be used for
germplasm conservation as well as for biochemical analysis [5].
For rapid multiplication of these wild plants, micropropagation is
being increasingly applied to supplement conventional methods of
propagation.
Our investigation is based using explants collected from mature
in vivo grown plant (about 2 years old) and the cultures were
maintained under continuous, cool and white fluorescent light
(2000 lux) during the whole experiment. In my opinion, the present
investigation would be the first thorough studies on organ cultures
of this taxon. As the tissues of mature plant are as a rule recalcitrant,
the tissue culture studies with explants taken from mature plant are
of great significance. Hence, the present studies were aimed at in vitro
regeneration of Solanum torvum through direct and callus mediated
shoot regeneration using explants taken from in vivo grown plant
(about 2 years old) under different hormonal regimes. Attempts were
also made to suggest methods for germplasm conservation through
tissue culture.
Materials and Methods
The experimental plant, Solanum torvum was procured from warm
moist fertile areas and was subjected to tissue culture experiments with
a view to exploring the possibilities of micropropagation protocol and
genetic upgradation through the use of somaclonal variants among
regenerated plants. This study was also aimed to develop protocol
for in vitro conservation of germplasm. The methodology of tissue
culture experiments includes the following steps:
(i) Preparation of culture media
(ii) Preparation of Explants
(iii) Inoculation and Transfer
(iv) Maintenance of Cultures
(v) Effect of Seasonal Variation on Regeneration and
(vi) Rooting and Transfer of Plantlets
Nutritional requirements for optimal growth of a tissue in vitro
may vary from species to species. Even tissues from different parts
of the same plant may have some specific requirements for their
satisfactory growth [6]. A wide range of culture media differing in
their elemental composition has been described in the literature. In
the present study, MS medium were used as basal medium as this was
suitable for regeneration and callus induction [7]. The medium was
prepared as such:
(i) Required quantities of agar (0.8% w/v) and sucrose (3% w/v)
were weighed out.
(ii) Sucrose was dissolved in some amount of distilled water to
give a concentrated solution and was filtered through the
Whatman filter paper No. 1 (9.0 cm) to remove the particulate
impurities, if any
(iii) Appropriate quantities of various stock solutions and growth
regulators were added.
(iv) Agar was dissolved in distilled water (in about ¼ of the
final volume of the medium) by heating in a water bath. The
dissolved agar solution & sucrose solution were mixed with
stock solution.
(v) The final volume of the medium was made upto 1 litre /
required volume with distilled water.
(vi) After proper mixing, the pH of the medium was adjusted to
5.8 using 0.1N NaOH or 0.1N HCl with the help of “Systronic”
digital pH meter model no. 335.
(vii) About 20 ml of the medium was poured into the culture tube
(25 x 100 mm)
(viii) The culture tubes were plugged with non-absorbent cotton
wrapped in cheese cloth. The cotton plugs were wrapped with
aluminium foils to prevent wetting during autoclaving.
(ix) The culture vessels were transferred to appropriate baskets
and autoclaved at 121oC for 20 minutes.
(x) Slants were prepared by keeping the tubes titled during
cooling.
The pH of the medium was adjusted to 5.8 before autoclaving
at 1210C for 20 minute. Various growth regulators and adjuvants
used as supplement of the basal medium were IBA, NAA, 2,4-D &
Kn. Stem segments (nodes and internodes) and leaf segments from
youngest shoots and shoot-tip segments collected from in vivo grown
mature plant (about 2 years old) of Solanum torvum during March
to November were used as explants and were surface sterilized.
These adjuvants were used in a wide range of concentration (1-10
mgl-1) either alone or in various combinations [8,9]. The stocks of
various growth regulators were prepared. All the precautions were
taken while sterilizing the tissues avoiding any damage to them. The
following steps were undertaken for sterilization of tissues or organ
explants.
(i) Washing the explatns in running tap water
(ii) The explants were treated for 2 min. in 1% cetavelon (cetrimide I.P. 20% w/v isopropyl alcohol B.P. 10% v/v)
solution followed by thorough washing in running tap water.
(iii) Washing and disinfecting the explants in 0.2% HgCl2 solution
for 3 to 5 min. depending upon the nature of the explants.
(iv) Further, washing them three or four times thoroughly with
sterile distilled water in an aseptic condition and
(v) Finally using sterile forcep, tissue explants were transferred to
sterile Petri dishes and were cut into required size with sterile
scalpel or blade. Usually, node and internode of 8-10 mm, leaf
segments of 5x5 mm and shoot-tip 10-15 mm were trimmed
out for explant preparation.
The cultures were incubated in culture room maintained at
25 + 2oC with a relative humidity of about 60% under continuous
fluorescent light (2000 lux, cool and white).
Calli obtained from different explants were taken out of the
culture tubes aseptically and kept in a presterilized. The callus was cut
into several pieces of almost equal sizes with the help of a sterilized
blade. Pieces of calli from the growing portions were inoculated into
the culture tubes containing MS medium with different combinations
and concentrations of growth regulators. The calli were incubated at
25+ 2oC for further growth and differentiation.
Microshoots (3-4 cm) obtained from shoot-tip, nodal segment
and regenerative callus in Solanum. torvum were cultured on MS
and rooting media (1/2 MS salts + full strength vitamins & amino
acid) supplemented with IBA and NAA singly and in combination
for rhizogenesis. Culture conditions were kept constant as in shoot
regeneration (Temp. 25 + 2oC, Light - 2000 lux, continuous, cool,
white and fluorescent).
Results and Discussion
In the present experimental system, nodal segment (8-10 mm),
internodal segment (8-10mm), shoot-tip (10-15 mm) and leaf (5x5
mm) of these sizes were taken for experimentation and these explants
yielded better results in culture. It was also remarkable in the present
system that a proper amount of growing callus was essential for
inoculum to had better differentiation and regeneration, a small piece
of callus having few cells could not survive in culture.
The composition of culture medium is the most important factor
for the establishment of tissue culture. It is confirmed that there is
no fixed combination of the medium which is suitable for all the
plants and even the different organs of the same plant. A particular
combination of the nutrient medium is suitable for a certain group of
plants but the same combination proves ineffective for other plants
[10,11]. So, the selection of proper culture medium is essential for
the tissue culture experiment of any plant. The response of two basal
media viz. MS and Nitsch was tested in case of present experimental
system and the results have been presented. MS medium was found
most suitable for shoot regeneration and callus growth. MS medium
was proved equally well in many other plants too. Normally, a high
cytokinin to auxin ratio promotes shoot formation while a higher
auxin to cytokinin ratio favours root differentiation [12]. In a number
of cases, cytokinin alone is sufficient for shoot regeneration and callus formation [13]. Identical response of cytokinin was encountered
in Solanum. torvum cultures. Kinetin (Kn) in the concentration of
2-3mgl-1 induced direct development of shoots from nodal and shoottip
segments in Solanum torvum, optimum response was obtained
on 2mgl-1 Kn. The frequency of shoot regeneration was better in
nodal culture of Solanum torvum than shoot-tip culture. No callus
formation was obtained on Kn supported media in nodal and shoottip
explants. Kn above 3mgl-1 had adverse effect on shoot regeneration
in nodal and shoot-tip cultures of Solanum toruvm [14,15].
In the present investigation, the best shoot regeneration in nodal
explant was obtained on 4mgl-1 Kn+2mgl-1 NAA and in shoot-tip
explants on 3mgl-1Kn+ 2mgl-1 NAA and 2mgl-1 2,4-D + 2mgl-1 Kn.
Shoot regeneration through callus subculture was frequent in the
present experimental system on NAA/ 2,4-D and Kn supplemented
media, the optimum response with better shoot regeneration from
callus was noted at on 5mgl-1Kn and 2mgl-1 NAA and 2mgl-1 2,4-
D + 4mgl-1Kn. This is also in conformity of the above facts. Thus, a
fine balance of exogenous auxin and cytokinin / cytokinin alone is
necessary before successful regeneration can occur.
Kn in combination with NAA / 2,4-D proved effective for
shoot regeneration and callus growth in this experimental system
[16,17]. The callus in general was greenish-white / white, compact,
hydrated and crystalline in appearance. However, in some hormonal
combinations, the node derived callus was creamy, white, compact,
hydrated and crystalline in appearance. Callus mediated regeneration
was frequent in sub culture on 2mgl-1 NAA and 3-5mgl-1 Kn / 2,4-
D+Kn. The optimum response of callus mediated callogenesis was
recorded on 5mgl-1 Kn + 2mgl-1 NAA and 2mgl-1 2,4-D + 4mgl-1
Kn. In addition to direct shoot regeneration in nodal and shoot-tip
explants, protocol for callus mediated shoot regeneration can also
be adopted in the present experimental system as these shoots were
morphologically identical to parent plants. The rejuvenation in callus
subculture was recorded on 2mgl-1 2,4-D + 2mgl-1 Kn, the callus
gradually turned brown in the beginning and after a month profuse
shining white callus grew from degenerated callus mass on the same
combination of hormones. Calli were maintained at 25+ 2oC in
culture till 1½ years for regeneration on 1mgl-1 NAA + 1mgl-1 Kn and
no regeneration was noted on maintenance medium (1mgl-1 NAA +
1mgl-1 Kn). The rejuvenation in callus subculture was recorded on
2mgl-1 2,4-D + 2mgl-1 Kn, the callus gradually turned brown in the
beginning and after a month profuse shining white callus grew from
degenerated callus mass on the same combination of hormones.
The best response for shoot regeneration was obtained at 25+ 2oC on 2mgl-1 Kn in nodal and shoot-tip cultures of Solanum torvum whereas NAA (2mgl-1) + Kn (2-4mgl-1) and 2,4-D (1-2mgl-1)+Kn (1-2mgl-1) were most responsive combinations for callus growth as well as shoot formation in the present system. In general, auxin and cytokinin above 5mgl-1 were found to be inhibitory for differentiation and regeneration [18]. Direct formation of shoots
was frequent in primary cultures of nodal and shoot-tip segments in Solanum torvum. Differentiation of callus was obtained on 2,4-D and NAA / 2,4-D + Kn combinations from nodal, internodal, leaf and shoot-tip cultures, callus mediated shoot regeneration was frequent in culture during the present investigation. Identical effects of auxin cytokinin combination on shoot multiplication / callus induction
were observed in many plants [19,20]. The role of sugars in tissue culture experiments was extensively studied. They provide energy source and maintain a minimum osmotic potential for the cultured tissue. Many carbohydrates have been used in tissue culture but among them sucrose is the most effective except in a few plants where glucose was found superior to sucrose [21]. Sucrose is generally used at the concentrations of 20-30 g/l and in the present investigation, 30 g/l sucrose was found most effective.
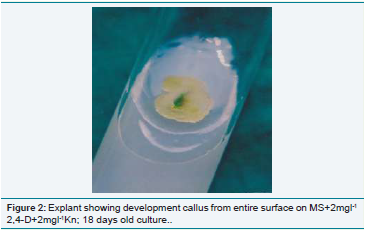
Figure 2: Explant showing development callus from entire surface on MS+2mgl-1
2,4-D+2mgl-1Kn; 18 days old culture..
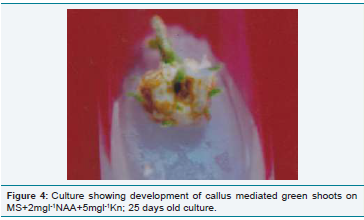
Figure 4: Culture showing development of callus mediated green shoots on
MS+2mgl-1NAA+5mgl-1Kn; 25 days old culture.
Conclusion
It was noticed that particular combination of medium was effective
for regeneration and growth of callus. Among phytohormones,
cytokinin was found to be more pronounced than auxin for callus
formation and callus was crystalline. The nodal culture was found to
be better than shoot tip culture.
Acknowledgements
The authors are extremely grateful to Dr. Santosh Kumar, Project
Coordinator, UGC-SAP (DRS-Phase–I), University Department
of Botany, B.R.A. Bihar University, Muzaffarpur for providing all
facilities. The authors feel highly indebted to Dr. Arvind Kumar Jha
whose comments and suggestions substantially improved the original
manuscript.