Research article
Fungal Spoilage of Stored Fruits ofCarica papaya L. and Vitis vinifera L. and Fungitoxicity of Plants Extracts
Deepti Srivastava1* and Nisha Misra2
1Department of Botany, S.V.M.M P.G. College, Gorakhpur- 273001, India
2Department of Botany, D.D.U. Gorakhpur University, Gorakhpur- 273009, India
Corresponding author: Deepti Srivastava, Department of Botany, S.V.M.M P.G. College, Gorakhpur- 273001,India; Tel: 9454580294; Email: srivastavadeepti2011@gmail.com
Citation: Srivastava D, Misra N. Fungal Spoilage of Stored Fruits of Carica papaya L. and Vitis vinifera L. and Fungitoxicity of Plants Extracts. J Plant Sci Res. 2017; 4(2): 170.
Copyright © Srivastava D. 2017 This is an open access article distributed under the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 4, Issue: 2
Submission: 21/08/2017; Accepted: 13/09/2017; Published: 03/10/2017
Abstract
The sample of fruits of Carica papaya L. and Vitis vinifera L. were collected from fruit vendors, fruit shops and mandies of Gorakhpur and bought to the laboratory in pre sterilized polythene bags. A total of 36 fungi associated with the fruits were isolated by standard method. Dynamics of rotting, test for pathogenicity, weight loss and per cent loss of protein, carbohydrate and phosphorus were observed. Dynamics of rotting were highest for Corticum rolfsii Sacc; Lasiodiplodia theobromae Pat; Pestalotia bicolor Ell and Ev; Pestalotia foedans Saccardo and Ellis and Trichothecium roseum Pers. Link. Ex: which also caused highest loss of weight, loss of protein, carbohydrate and phosphorus contents of all the fruits? Extract of 47 angiospermic plants were tested for their efficacy against Corticum rolfsii, Lasiodiplodia theobromae, Pestalotia bicolor, Pestalotia foedans and Trichothecium roseum by inverted plate method. Erythrina indica L., Foeniculum vulgare Mill and Spillanthus acmella exhibited 100 per cent efficacy. The Minimum Inhibitory Concentration (MIC) was 5000 ppm.
Keywords:
Fruits; Pathogenicity; Rotting; Plant extracts; MIC
Introduction
Plants suffer severely from post-harvest diseases. These diseases develop on plant produce or plant products during harvesting, grading, packing and transportation to market up to consumers and while the produce is in possession of the consumer until the movement of actual consumption are huge. During this period plant products attacked by micro organisms show various symptoms. One of the frequently occurring symptoms is rot. In many cases micro organisms secrete toxic substances that make the remainder of the product unfit for consumption or lower it’s nutritional and sale values [1-5].
Fruits are an important part of our food from pre historic time. They are chief source of vitamins, minerals, protein, carbohydrate, fat, fiber and other minor and major elements. Ripe fruits are susceptible to attack by rot micro organisms because at this stage they contain enough moisture and highest nutritional contents which make them more susceptible for the growth of fungi.
The present paper describes the rot disease of fruits of Carica papaya L. and Vitis vinifera L. caused by fungi during storage and their effect on carbohydrate, protein and phosphorus contents. The paper also describes fungitoxicity of some extracts of plant parts.
Materials and Methods
Sample of fruits of Carica papaya and Vitis vinifera were collected periodically from fruit vendors, fruit shops and mandies as well as from the markets of nearby areas in pre sterilized polythene bags and brought to the laboratory. Symptoms of disease and associated mycoflora were observed.
Isolation of the associated mycoflora
Isolation of associated mycoflora with spoiled fruits was done after their surface sterilization with 90 per cent alcohol. The isolated fungi were transferred to Czapek-Dox Agar (CDA) medium. Some isolations were made by transferring the hyphae directly from the aerial mycelium present over the surface of the infested fruits.
Petri plates were incubated at the temperature of 24 ± 2 °C. During the incubation period, petri plates were examined daily from third day of the incubation for the fungi. All the fungi, thus isolated were purified by single spore technique. The pure cultures were maintained on CDA slants at 10 °C. The cultures were identified with the help of available literature.
Czapek-Dox Agar (CDA) medium was used to isolate the fungal flora and to observe its growth during the course of investigation. Potato Dextrose Agar (PDA) and Oat Meal Agar (OMA) media were used to test antifungal activity of leaf and seed extract against test fungi.
Test for pathogenicity
Pathogenicity tests were conducted to confirm the pathogenic nature of the isolated fungi on their respective hosts. Fresh and sound fruits of Carica papaya and Vitis vinifera were surface sterilized with 90 per cent alcohol to remove the superficial mycoflora as well as to maintain the natural nature of the skin of fruits. An injury of 10 mm depth was made over the surface of the fruits with the help of sterilized cork borer of 5 mm diameter. A bit of tissue was taken out and three day old inoculum was placed in the pit. The piece of the fruit tissue taken out was inserted back to its position and the wound was then sealed with the sterilized cotton.
The inoculated fruits were placed in sterilized glass jars at the temperature of 24 ± 2 °C.
The pathogenicity of the organism was considered established only when Koch’s postulate was fully satisfied. For both the fruits five replicates were maintained.
Data were also recorded for the dynamics of rotting using following formula of Bottcher (1986) [6,7].
Y = β1 (x-z)2
Where,
x = duration of storage in days
Y= rot
β1 = Linear rise
z = a period without macroscopic symptoms
Weight loss
To observe change in the weight of the fruits due to the infection caused by the pathogenic fungi, fresh and healthy fruits were surface sterilized and inoculated separately with respective pathogenic species as described above.
Similar control sets were maintained in which the pathogenicfungi were not inoculated.
Weight loss was noted after incubating the controlled andinoculated sets for a week at 24 ± 2 °C . Loss in weight was determinedby following formula:
Weight loss = W - w / W x 100
Where,
W = weight of the infested fruit before incubation
w = weight of the infested fruit incubation
Estimation of chemical constituents of fruits inoculated bytest fungi
Methods of Lowry et al. (1951), Dreywood (1946) and Fiske et al. (1925) were followed to study the effect of test fungi on protein, carbohydrate and phosphorus (mineral content) respectively. Fresh fruits nearly of the same maturity were surface sterilized by 90 per cent alcohol and were inoculated separately by three day old cultures of test fungi on respective fruits. The inoculated fruits were placed in sterilized glass jars at 24 ±2 °C temperature for one week. Control sets of sterilized and uninoculated fruits were also prepared similarly. Protein, carbohydrate and phosphorus (mineral content) of both inoculated and uninoculated (control) fruits were estimated [8-12].
Screening of vapour of plant parts for their fungitoxicityagainst test fungi
Some plants belonging to different families of angiosperms were collected from Gorakhpur district. These were identified with the help of various flora.
Freshly collected parts of each plant (especially leaves and seeds) were surface sterilized by dipping in 0.1 per cent mercuric chloride as it is a good disinfectant and were then washed with sterile water. The plant parts (100 g) were crushed into pieces and macerated with 100 ml of sterile water (1:1 w/v) in mortar with the help of pestle. To obtain the extract the pulp was squeezed through a double layered muslin cloth. 2.0 mg streptopenicillin was added to the extract to prevent bacterial activity during the course of experimentation. The extract was then assayed for its volatile anti fungal activity against the test fungi by inverted plate method [13-17].
10 ml of medium was aseptically poured into each of the sterilized Petri plates of 7.5 cm diameter. One assay disc of 5 mm diameter of fungi, taken from periphery of one week old culture, was inoculated in Petri plates. The inoculated Petri plates were turned upside down. To the lower lid of the Petri plate, 5 ml of extract soaked in sterilized cotton was aseptically inoculated. A control set was prepared similarly, using cotton soaked in 5 ml of distilled and sterile water instead of filtrate. The Petri plates were incubated for six days at 24 ± 2 °C.
On the seventh day, diameter of colony of treatment as well as control sets was measured in mutually perpendicular direction. The experiment contained five replicates and was repeated thrice. All other experiments were set likewise, unless mentioned otherwise. Per cent inhibition of mycelium growth was calculated on mean value of colony diameter using the formula of Vincent (1947) [18-22].
% inhibition of mycelia growth = dc-dt / dt x 100
Where,
dc = Average diameter of fungal colony in control sets
dt = Average diameter of fungal colony in treatment sets
Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentration was also observed by inverted Petri plate method as described above. For MIC 1 to 10 ml (1000-10,000 ppm) extract of Erythrina indica L., Foeniculum vulgare L. and Spillanthus acmella Murr. Soaked in sterilized cotton were introduced in the lower lid of separate petri plates. The Petri plates were incubated for six days at 24 ± 2 °C. On the seventh day, diameter of colony of treatment as well as of the control sets was measured by the formula of percent inhibition of mycelia growth.
Result and Discussion
A survey of local markets as well as of the markets of nearbyareas was periodically conducted for two years. During this perioda number of soft rot causing fungi were collected from the fruitsof Carica papaya and Vitis vinifera. Symptoms of the diseasesencountered and the morphological characters of the fungi isolatedwere taken into consideration for their identification. The results aregiven in Tables 1-6.
Table 5: Showing screening of vapours of extracts of some plants for their activity against mycelial growth of test fungi.
Table 6: Showing minimum inhibitory concentration of the extracts of Erythrina indica, Foeniculum vulgare and Spillanthus acmella against test fungi.
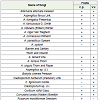
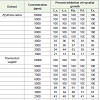
Out of 36 fungi isolated from fruits of Carica papaya and Vitis vinifera, 31 were associated with Carica papaya (Table 1). Among them 20 species, viz., Aspergillus fumigatus, A. funiculosus, A. nidulans, A. ochraceous, A. parasiticus, A. tamari, A. terreus, Aspergillus sp. U.I., Mucor racemosus, Pestalotia bicolor, P. foedans, Penicillium chrysogenum, P. oxalicum, Sterile mycelium black, greenish black and Sterile mycelium white are new record from this fruit. 26 fungi were observed to be associated with Vitis vinifera (Table 1). Among them, 21 fungi viz., Aspergillus flavus, A. funiculosus, A. nidulans, A. ochraceous, A. sydowi, A. tamari, A. terreus, A. unguis, Aspergillus sp. U.I., Cladosporium herbarum, C. lignicolum, Cladosporium sp., U.I., Corticum rolfsii, Lasiodiplodia theobromae, Mucor racemosus, Pestalotia bicolor, P. foedans, Penicillium citrinum, P. oxalicum, Sterile mycelium (greenish black) and Trichothecium roseum have not been reported earlier from this fruit.
The result in Table 1 also shows that Alternaria alternata, Aspergillus flavus, A. niger, Corticum rolfsii, Lasiodiplodia theobromae, Pestalotia bicolor, P. foedans, Rhizopus stolonifer and Trichothecium roseum were associated with both the fruits. However certain species were specific for any one fruit eg. Aspergillus fumigatus, A. parasiticus, Fusarium moniliforme, F. oxysporum, F. semitectum, F. solani, Fusarium sp. U.I., Penicillium chrysogenum, sterile mycelium black and sterile mycelium white were found to be associated only with fruit of Carica papaya. Whereas Aspergillus sydowi, A. unguis, Botrytis cinerea, Cladiosporium lignicolum and Cladosporium sp. U.I., were specific for Vitis vinifera. The specific appearance of some fungi on any one fruit may be because of their ability to secrete different types of enzymes [19-25].
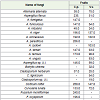
The result in Table 2 shows dynamics of rotting by different fungi during storage of fruits. For the fruits of Carica papaya it was highest by Rhizopus stolonifer (288) followed by sterile mycelium greenish black (262), Sterile mycelium black (256), Aspergillus parasiticus (256), Corticum rolfsii (250) and least by Alternaria alternate (38).
In case of fruits of Vitis vinifera dynamics of rotting was highestby Trichothecium roseum (172) followed by Lasiodiplodia theobromae (147), Corticum rolfsii (147), Aspergillus niger (137), Sterile mycelium greenish black (130) and it was least by Penicillium citrinum (14).
Pathogenicity test were conducted to confirm the pathogenic nature of isolated fungi on their respective hosts. The effect of pathogenic fungi on the weight of fruits was also noted. The result is given in Table 3.
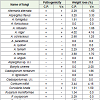
Out of 31 fungi isolated from Carica papaya, 21 species confirmed their pathogenic nature and loss in weight was noted to be 4.78, 4.59, 4.02, 3.83, 3.82, 3.63, 3.44, 3.25, 2.87, 2.87, 2.68, 2.30, 2.29, 2.29, 2.29, 2.10, 1.91, 1.91, 1.72, 1.34 and 1.34 per cent when inoculated by Lasiodiplodia theobromae, Trichothecium roseum, Aspergillus niger, Pestalotia foedans, Penicillium citrinum, Aspergillus terreus, Corticum rolfsii, Aspergillus flavus, Mucor racemosus, Penicillium chrysogenum, Aspergillus ochraceous, Pestalotia bicolor, Alternaria alternate, Aspergillus tamarii, Fusarium oxysporum, Rhizopus stolonifer, Aspergillus fumigatus, Curvularia lunata, Fusarium semitectum, Aspergillus nidulans and Fusarium moniliforme.
The fruits of Vitis vinifera were infested by 26 species of which 18 species were found to be pathogenic, Loss in weight was noted to be 4.16, 4.16, 3.33, 3.33, 3.00, 2.50, 2.50, 2.00, 2.00, 2.00, 1.70, 1.66, 1.66, 1.66, 1.60, 1.50, 1.25 and 1.25 per cent when inoculated by Aspergillus niger, Corticum rolfsii, Aspergillus flavus, Trichothecium roseum, Lasiodiplodia theobromae, Mucor racemosus, Rhizopus stolonifer, Aspergillus tamarii, Botrytis cinerea, Pestalotia bicolor, Aspergillus terreus, A. nidulans, Cladosporium herbarum, Pestalotia foedans, Alternaria alternata, Curvularia lunata, Aspergillus ochraceous and Penicillium citrinum.
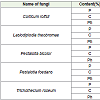
From the Table 4 it may be noticed that the protein content of Carica papaya decreased up to 0.19, 0.14, 0.11, 0.08 and 0.06 per cent when it was inoculated by Lasiodiplodia theobromae, Trichothecium roseum, Pestalotia foedans, Corticum rolfsii and Pestalotia bicolor respectively. The percentage of carbohydrate decreased up to 4.08, 2.16, 1.68, 1.32 and 1.08 per cent when inoculated by Lasiodiplodia theobromae, Corticum rolfsii, Pestalotia bicolor, P. foedans and Trichothecium roseum respectively and the percentage of phosphorus decreased up to 7.0, 5.0, 3.0, 3.0 and 1.0 per cent when inoculated by Pestalotia foedans, Lasiodiplodia theobromae, Pestalotia bicolor, Trichothecium roseum and Corticum rolfsii respectively [26].
In Vitis vinifera the loss in protein content was noted to be 0.32, 0.27, 0.22, 0.14 and 0.11 percent when inoculated with Trichothecium roseum, Lasiodiplodia theobromae, Pestalotia foedans, P. bicolor and Corticum rolfsii respectively, the percentage of carbohydrate decreased up to 7.38, 4.50, 1.38, 1.26, and 0.42 per cent when inoculated by Pestalotia foedans, Corticum rolfsii, Trichothecium roseum, Lasiodiplodia theobromae and Pestalotia bicolor. Loss in phosphorus content was up to 22, 20, 10, 6.0 and 2.0 per cent when inoculated by Lasiodiplodia theobromae, Pestalotia foedans, P. bicolor, Trichothecium roseum and Corticum rolfsii respectively.
The result of screening of vapors of plant parts (leaves and seeds)given in the Table 5. The plant species are grouped alphabetically under their respective families.
It is evident from the data in Table 5 that aqueous extracts of different parts of 46 plant species belonging to 34 families of angiosperms screened against test fungi, most of the species showed either poor (below 50%) or moderate (above 50%) activity. Erythrinaindica L., Foeniculum vulgare L. and Spillanthus acmella Murr, were found to exhibit absolute toxicity against test fungi [27].
It is clear that extract of Erythrina indica, Foeniculum vulgare and Spillanthus acmella after in vivo studies may be used for the preservation of fruits during storage. However the active ingredient and its isolation need further attention. The anti fungal activity of the extract against Lasiodiplodia theobromae, Corticum rolfsii, Pestalotia bicolor, Pestalotia foedans and Trichothecium roseum is being reported for the first time.
Conclusion
Some healthy plants and their parts show antifungal and microbial activity because they have some naturally occurring substances which play an effective role in plant disease resistance. A few of these substances have been purified, characterized and exploited in phytochemotherapy.
Acknowledgement
Authors are thankful to the Head of the Botany department, D.D.U. Gorakhpur University, Gorakhpur for providing laboratory facilities.
References
- Amiri A, Bompeix G (2005) Diversity and population dynamics of Penicillium spp. on apples in pre- and post harvest environment: consequences for decay development. Plant Pathol 54: 74-81.
- Asthana A, Chandra H, Dikshit A, Dixit SN (1982) Volatile fungitoxicant from leaves of some higher plants against Helminthosporium oryzae. J Plant Dis Prot 89: 475-476.
- Bailey LH (2016) Manual of cultivated plants most commonly grown in continental United States and Canada (Classic Reprint). Fb &C Limited, pp. 1122.
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C (1968) Screening of Indian plants for biological activity: I. Indian J Exp Biol 6: 232-247.
- Paech H, Tracey MV (1955) Modern methods of plant analysis. Springer-Verlag, Berlin, 2: 1-611.
- Booth C (1971) The genus Fusarium. Commonwealth Mycological Institute, Surrey, England, pp. 237.
- Boettcher H (1986) Occurrence and intensity dynamics of parasitic rotting in stored vegetables. Arch Phytopathol Plant Prot 22: 465-481.
- Chandra H, Dikshit A (1981) Volatile fungitoxicant from leaves of Ageratum conyzoides against Colletotrichum capsici and Penicillium italicum. J Ind Bot Soc 60: 13-14.
- Domsch KH, Gams W, Hudson PS (1974) Fungi in agricultural soils. Q Rev Biol Vol: 49.
- Booth C (1971) Methods in microbiology, (Vol 4). Academic Press, London and New York, pp. 1-751.
- Ellis MB (1976) More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, CABI Publishing Series, pp. 507.
- Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66: 375.
- Barron GL (1968) The genera of hyphomycetes from soil. University of Minnesota, Williams & Wilkins, pp. 364.
- Gilman JC (2008) A manual of soil fungi. Chawla offset printers, Biotech books, Delhi, pp. 1-363
- Granger K, Horne AS (1924) A method of inoculating the apple. Ann Bot 38: 212-215.
- Kumar GR (1968) Flora nainitalensis: A hand book of the flowering plants of Nainital. Navayug Traders, New Delhi, pp. 1-529.
- Gupta S, Banerjee AB (1970) A rapid method of screening antifungal producing plants. Indian J Exp Biol 8: 148-149.
- 18. Lowry OH, Rosenbrongh NJ, Farr AL, Randull RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193: 265-275.
- Maheshwari JK (1963) The flora of Delhi. Council of Scientific & Industrial Research Publication, New Delhi, pp. 1-447.
- Nene YL, Thapliyal PN (1965) Antifungal properties of Anagaliis arvensis L. extracts. Nat Sci 52: 89-90.
- Raper KB, Thom C (1949) A manual of penicillia. William and Wilkins Co, Baltimore.
- Santapau H (1967) The flora of Khandala on the Western Ghats of India. Scientific Publishers, 16: 1-372.
- Srivastava TN (1976) Flora Gorakhpurensis. Cornell University, Today and Tomorrow’s Printers and Publishers, New Delhi, pp. 411.
- Srivastava D, Kumari A, Misra N (2009) Fungal spoilage of some fruits and screening of some leaves for fungitoxicity. Proc Nat Acad Sci Sect B, 79: 421-430.
- Subramanium CV (1971) Hyphomycetes: An account of Indian species, except Cercosporae. The University of Wisconsin – Madison, Indian Council of Agricultural Research, New Delhi, pp. 930.
- Thapliyal PN, Nene YL (1967) Inhibition of plant pathogen by higher plant substances. J Scient Indust Res 26: 289-299.
- Vincent JM (1947) Distortion of fungal hyphae in the presence of certain inhibitors. Nature 159: 850.