Research article
Improvement of Cocoyam (Xanthosoma sagittifolium L. Schott) Minitubers Production under Treatment of Glomus intraradices and Gigaspora margarita and their Impact on some Secondary Metabolite contents
Djeuani AC1,2*, Mbouobda HD1,3, Fotso1,3, Niemenak N1 and Omokolo ND1
1Department of Biological Sciences, Laboratory of plant physiology, Higher Teacher Training College (HTTC), University ofYaoundé I, Yaoundé-Cameroon
2Department of Plant Biology, Faculty of Sciences, University of Yaoundé, Yaoundé-Cameroon
3Department of Biology, Higher Teacher Training College, Bambili (HTTC), The University of Bamenda, Bamenda-Cameroon
Corresponding author: Djeuani AC, Department of Biological Sciences and Plant Biology, Laboratory of plant physiology, Higher Teacher Training College (HTTC), University of Yaoundé I, Yaoundé-Cameroon; Tel: +237 772 569 97 / +237 994 288 84; E-mail: djeuani.uyi@hotmail.com
Citation: Djeuani AC, Mbouobda HD, Fotso, Niemenak N, Omokolo ND. Improvement of Cocoyam (Xanthosoma sagittifolium L. Schott) Minitubers Production under Treatment of Glomus intraradices and Gigaspora margarita and their Impact on some Secondary Metabolite contents. J Plant Sci Res. 2017; 4(2): 169.
Copyright © Djeuani AC. 2017. This is an open access article distributed under the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 4, Issue: 2
Submission: 21/06/2017; Accepted: 27/07/2017; Published: 13/09/2017
Abstract
The use of arbuscular mycorrhizae in improving growth and production in plants is increasing. The effect of Glomus intraradices and Gigaspora margarita has been evaluated on the improvement of growth and tuberization in white cocoyam. The intake of mycorrhiza significantly increased the root number in Gi. margarita from 16.40±0.54 at D120, and for the leaf area 40.84±12.62cm2. The fresh and dry biomass and the mycorrhizal dependence increased progressively over time and are more significant in the presence of G. intraradices and Gi. margarita, 42.50±5.87 g; 15.89±4.07 g and 20.65% and 38.11±2.57 g; 15.89±2.35 g and 20.96% respectively at D180. The increase frequency of mycorrhization evaluated was 47% and 44% in G. intraradices and Gi. margarita. For all treatments applied, total chlorophyll content decreased over time. The presence of mycorrhizae almost completely doubled the number of minitubers obtained in the control (17 minitubers), recording 37 minitubers in G. intraradices and 32 minitubercules in Gi. margarita. On the biochemicalapproach, the application of AMF has a positive effect on the variation in the content of the metabolites studied compared to control. The results showed that when the total soluble sugar, total protein and total phenol contents decreased, the total amino acid and proline levels increased significantly (P<0,05) over time. Significant peaks were observed with Gi. margarita in the leaves 2.19±0.01 mg.g-1 of FW for total soluble sugar on D60; 2.99±0.09 mg.g-1 of FW for total soluble protein on the D60 and D120 respectively and 23.93±0.04 mg.g-1 of FW for the total phenols at day 60. The results obtained showed that cocoyam is a mycotrophic plant.
Keywords:
Xanthosoma sagittifolium L. Schott; Arbuscular mycorrhizal fungi; Minituberization and secondary metabolites
Introduction
Arbuscular Mycorrhizal Fungi (AMF) is mandatory symbionts of the vast majority of terrestrial plants [1-3]. Their application in agriculture is a sustainable and innovative solution. They represent an unavoidable concern in the progression towards less polluting “alternative agriculture” whose products are free from chemical residues [4]. Studies showed that AMFs are strongly involved in the acquisition of important nutrients of plant in soil. AMFs play a key role in improving the uptake of water in plants, contribute to the increase in antioxidant activity, to osmotic adjustment, improve hormone regulations, biological control of plant pathogens, soil fertility, plant nutrition, improve absorption and translocation of mineral nutrients, mainly Phosphorus (P), Nitrogen (N), Sulfur (S), Potassium (K), Calcium (Ca), Iron (Fe), Copper (Cu) and Zinc (Zn) and various trace elements from the soil to host plants, due to an extensive hyphae network of the soil, which propagates from roots colonized in the soil [5-12]. However, the use of arbuscular mycorrhizal fungi as an inoculum in agriculture remains promising and may be very important in addressing phosphorus deficiency problems in some plants that are highly dependent on their presence in the soil, such as plants with roots and tubers among which the most important are: irish potato (Solanum tuberosum L.), cassava (Manihot exculenta), taro (Colocasia exculenta L. Schott) and cocoyam (Xanthosoma sagittifolium L. Schott) [13,14].
Cocoyam (Xanthosoma sagittifolium L. Schott) is an important carbon source for food security in Cameroon. It is grown for its tubers and leaves which constitute a source of starch and amino acids [15]. Its cultivation requires the presence of Phosphorus (P), Nitrogen (N) in the soil. Its tubers have higher protein content than cassava [16]. Tubers of cocoyam are moderately richer in water-soluble vitamins [17]. The consumption of tubers of red cocoyam is strongly recommended to diabetics because of its richness in insulin [18]. These tubers are widely used in the agro-food industries, as in Latin America, where they are raw materials for the manufacture of meals and feed for children [15]. Despite its place in human consumption and the possibilities it offers for the export of tubers, the intensive culture of cocoyam is slowed down by various bacterial, fungal and viral infections and by the unavailability of planting material that is sufficient and free from disease [19]. Strategies to improve the cocoyam cultivation by conventional genetic crossing methods have not given expected results due to the narrowness of the genetic diversity of existing varieties [20]. Application of biotechnology to facilitate production of cocoyam vitroplants, microtuberization or in vitro production of tubers and minituber production are already tested alternatives [21-23]. The use of arbuscular mycorrhiza could be one way to improve cocoyam cultivation. There is no available data on the influence of AMFs on Araceae production. Nevertheless, the work of and showed that in Solanum tuberosum, for example, the use of Arbuscular Mycorrhizal Fungi (AMF) had a positive impact on improving growth, increasing tuber size and increasing field yield [24,25]. Similarly, have also shown that an in vitro application of Rhizophagus irregular is would currently be an important means of increasing yields in cassava [26]. Furthermore, for an application of Glomus etunicatum, Glomus mosseae and Gigaspora sp., accelerated growth and improved tuber yield in Dioscorea spp. AMF inoculation not only promotes the growth of plants, they establish a symbiotic relationship with plant roots and influence directly or indirectly the host’s metabolic processes [27,28]. AMF induce different changed in the production of secondary metabolites in plant [29]. That change in plant secondary metabolite concentrations of AM plants may be due to enhanced mineral nutrition, and/or is a result of plant reaction to fungal colonization [11]. It is in this logic that the general objective of this study was to evaluate the effect of Glomus intraradices and Gigaspora margarita on cocoyam minituberization highlighting their influence on some plant metabolites.
Materials and Methods
Plant material
The plant material constitutes of cocoyam vitroplants obtained through in vitro culture of the apex of white cultivar harvested from the experimental farm [30,23]. After 30 days of acclimatization; the plantlets were thus ready for the different treatments.
Inoculation, treatment and morphological analysis on cocoyam mycorrhizae plantlets
Once acclimated, the cocoyam seedlings were divided into three lots of 50 each, representing Glomus intraradices, Gigaspora margarita treatments and control. For the mycorrhizal plantlets, 30 g of each inoculum was applied for each single cocoyam plant. The various treatments were placed under a shade and then separated by a distance of 0.5 m. Watering every two days with water, the plants received every 10 days a mineral solution consisting of the medium of Murashig and Skoog without phosphate component. To evaluate the potentialities of the treatments applied to induce tuberization, 5 plantlets of cocoyam seedlings were chosen at random every 60 days until 180 days, in each treatment and in which the number of roots, the number of leaves, the leaf area, seedlings, total fresh biomass and total dry biomass, mycorrhizal dependence were assessed. On day 180 the harvest took place due to total yellowing of the leaves of the treated plantlets. From harvested minitubers, their number, percentage of tuberization, average number per plants, average weight per plants, and increase rate in the weights of minitubers, average height and diameter of minitubers were measured according to the different treatments and compared to the control.
Histological Analysis
Determination of root colonization
This was carried out by the method established by and then modified [31,32]. The percentage of total root length colonized by AMF was assessed by cutting a random sub-sample of 1 g of fresh roots into 0.5 to 1.0 cm segments. Root segments were cleared in 10 g.L-1 KOH for 15 min at 90°C, then bleached with H2O2 (10%) for 3-6 hours. Rinsed with water acidified with HCl (10%) and stained with 5 ml of blue ink of trade prepared in alcohol vinegar at 8% for 10-30 min at 90°C. Mycorrhizal colonization was determined as described by [33].
Biochemical Analysis
Chlorophyll content
Chlorophyll was extracted from mycorrhizal and non-mycorrhizal leaves of cocoyam seedlings following the method of [34]. In fact, 0.5 g of leaves for each treatment was ground in the presence of sterile fine sand in a porcelain mortar. 15 ml of 80% acetone was added and incubated overnight in a dark place. The solution was then centrifuge at 5000 g for 5 min. The supernatant was collected and the residue was homogenized with 80% acetone and centrifuged. The content of chlorophylls a,b and total chlorophyll was estimated by the UV-visible spectrophotometer at 645, 663 and 652 nm against a blank consisting of 80% acetone solution and according to the method of [34].
Total soluble sugars and total amino acids contents
The extraction of total soluble sugars and amino acids from rhizomes and leaves of cocoyam plantlets was realized according to the method described by [35]. 1 g of fresh matter was crushed in a porcelain mortar with fine sand + 10 mL of 80% alcohol and centrifuged at 5000 g for 30 min after 30 min of incubation. For total soluble sugar analysis, each sample was made of 5 mL of the anthrone solution and 50 μL of the plant extract. The mixture was incubated in a water bath at 80°C for 20 min. After cooling, the absorbance of the complex green formed was read at 620 nm. The sugar content was evaluated with reference to a standard curve established with glucose solution (1 mg.mL-1) in alcohol at 80°C and expressed in mg.g- 1 of Fresh Weight (FW). For amino acids analysis, 30 μl of alcoholic extract was mixed with 0.5 ml citrate buffer (0.2 M pH 5) and 1 mL acetonic ninhydrine solution (1% + 0.06% KCN) then placed in a boiling water bath for 15 min. After cooling at room temperature, 8 ml of distilled water was added to it. The absorbance of complex violet blue formed was measured at 570 nm. For each extract, three readings were done and the content in amino acid expressed in mg.g-1 of Weight of Fresh (WF), using glycine as standard.
Proline contents
The proline is measured according to the method of [36]. It consists of taking 20 mg of the plant material and then adding 2 ml of 40% methanol, the whole is heated at 85°C, in a water bath for one hour. After cooling, 1 ml of the extract is removed, to which are added: 1 ml of acetic acid; 25 mg of ninhydrin solution the mixture obtained is boiled for 30 minutes at 100°C. The solution turns red. After cooling, 5 ml of toluene are added with stirring, two phases appear, the upper phase containing the proline was recovered and its optical density was determined at 528 nm. The values obtained are converted into proline levels by means of a previously established standard curve from a series of proline concentration solutions ranging from 0 to 1 mg.ml-1. The proline content was expressed in mg.g-1 of Fresh Weight (FW).
Total soluble proteins contents
Leaves or roots (1 g) were ground in chilled mortar with 2 mL of TAMET buffer (0.5 M Tris, 0.3 M ascorbic acid, 0.2% (v/v) β-mercaptoethanol, 0.01 M EDTA and 0.02% (v/v) Triton X 100, pH 6.7) and 0.125 g Polyvinylpyrrolidone (PVP) was added in the medium. The crude homogenate was centrifuged for 15 min at 5000 g and 4°C after incubation. The supernatant was removed and used as crude extract for proteins and enzymes assays. Proteins were quantified according to method. The total soluble proteins content was expressed in mg.g-1 of fresh weight (FW) [37].
Phenols content
Phenolic compounds were extracted as described by [38,39]. 1 g of leaves or roots of cocoyam were ground in chilled mortars with 2 ml of 80% (v/v) methanol at 4°C. After incubation, tubes were centrifuged thrice at 7000 g for 30 min, supernatant were recuperated each time. Mixture of the three supernatants constituted the crude extract. Total phenols were quantified using the method described by [40]. 15 μl of alcoholic extract were added to Folin-Ciocalteu reagent (250 μl), 2.5 mL of distilled water and 0.5 ml of sodium carbonate (20%). The mixture was incubated at 40°C for 20 min and the blue color was determined at 760 nm. The content of soluble phenolic was expressed in mg-equivalent of gallic acid per Fresh Weight (FW).
Statistical Analysis
Results were expressed in for of Means ± SD, analysis were done using excel for the treatment and realization of curves and histograms. Duncan’s test with the least significant difference of 5 % (P<0.05) were used to compared results following treatments over time with SPSS 20.0.
Results
Evaluation of growth parameters of cocoyam under different treatments
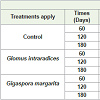
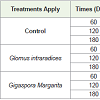
Morphological analyzes carried out during the growth and induction of minituberization of cocoyam showed that the Glomus intraradices and Gigaspora margarita strains used had a significant effect (P <0.05) on the different growth parameters evaluated (Table 1). It is noted that the maximum average root number obtained was 16.40±0.54 in the presence of Gi. margarita after 120 days of growth. This number decreases to 10.60±3.84 after 180 days. In Control and in G. intraradices, the number of roots remains significant during the first 60 days of growth, 14.40±1.51 and 15.60±2.60 respectively. Mycorrhization promoted root development in general (Figure 1). For the mean leaf number, mean leaf area, average seedling size, the results showed that they evolved in the form of the gauss curve with a maximum observed on D120 whatever the treatment applied. The mean number of leaves was 2.52±0.73, 2.54±0.61 2.56±0.81 in control, G. intraradices and Gi. Margarita respectively. These results showed no significant difference in term of number of leaves under different treatments (Table 1). Whereas on the leaf area there was a significant increase between D60 and D120, either 39% in the control, 24.90% in G. intraradices or 42.50% in Gi. margarita. No significant difference in seedling size in the control pleat was recorded. Nevertheless, this size of cocoyam seedlings, increased significantly by 38% and 27.93% between D60 and D120, then decreased between D120 and D180, respectively in both G. intraradices and Gi. margarita (Table 1).
Figure 1: Aspect of cocoyam plantlets and roots mycorrhizae with G. intraradices and Gi. margarita compared to control. The appearance of seedlings: control (A) G. intraradices (B) and Gi. margarita (C) Roots coating the substrate: control (D) G. intraradices (E) Gi. margarita (F) Roots of cocoyam for each treatment apply: control (G) G. intraradices (H) Gi. margarita (I).
Evaluation of fresh and dry biomass of cocoyam under different treatments
Analyzes of fresh and dry biomass showed an increase over time (Table 2). Mycorrhization significantly improved the fresh weight of the seedlings compared to the control. The maximum of the significant values was obtained on D180, either 32.04±4.35 g in the control, 42.50±5.87 g in G. intraradices and 38.11±2.57 in Gigaspora margarita for the fresh weight respectively. Similarly, dry biomass values were significant at D180, with 11.61±1.17 g, 13.88±4.07 g and 15.89±2.35 g for the control, G. intraradices and Gi. Margarita respectively.
Table 2: Fresh and dry matter content of cocoyam plants in response to mycorrhization during minituberization and determination of the mycorrhizogenic capacity of each treatment.
Evaluation of mycorrhizal parameters and different structures of arbuscular mycorrhizae observed on the roots of plants cocoyam
For the evaluation of the establishment of symbiosis between the cocoyam seedlings and the mycorrhizal biofertilizing strains used, the histological analysis, showed no mycorrhization in the control (Figure 2). Mycorrhizal structures were observed in roots. The significant values are obtained on D120, either 47%; 16.1%; 14. 95%; 34.47% and 2.40% in Glomus intraradices and 44%; 4.04%; 13.7%; 9.18% and 0.55% in Gigaspora margarita respectively or the frequency of mycorrhization (F%), root system mycorrhization intensity (M%), arbuscule content (a%), mycorrhizal intensity in mycorrhizal fragments (m%) and abundance of arbuscules in the root system (A%). Results showed that the mycorrhizal status varies over time and also evolves in GAUSS curve (Table 2). The intensity of mycorrhization in mycorrhizal fragments is very important in G. intraradices, while the abundance of arbuscular in the root system is very low in the presence of Gi. margarita, or even less than 1.
Figure 2: Mycorrhizal structures observed in the cocoyam cells root. Control (A) Structure of hyphae: G. intraradices (B) Gi. margarita (C) Arbuscular structures: G. intraradices (D) Gi. margarita (E) Cortex (c), roots cells of the hypodermis (ch), roots cells of the epidermis (ce), extracellular hyphae (he), hyphae (h) and arbuscules (a) Observed at 400X by optical microscope.
Harvest of cocoyam minitubers under treatments of G. intraradices and Gi. margarita
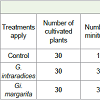
The contribution of G. intraradices and Gi. margarita to improve growth and tuberization in cocoyam, has significant results (P <0.05) (Table 3 and Figure 3). These minitubers presented sizes which varying according to the treatments (Figure 3).Mycorrhization necessarily induced the production of minitubers, from 17 minitubercules to control almost double the value of 37 minitubercules in G. intraradices and 32 in Gi. margarita. Despite the fact that the percentage of tuberization is very close to 50% for all the applied treatments, it is noted that the average number of minitubers per plant has increased significantly 1.23±0.76 in G. intraradices and 1.06±0.67 at Gi. margarita compared to control 0.56±0.18. At 5%, that there is a significant difference for the average mass of minitubers per plant compared to control, the important values which are obtained with G. intraradices (2.84±1.14 g) and Gi. margarita (2.60±1.38 g), showing a mass increase rate of 44.01 and 38.84% compared to the control (Table 3). Similarly, the size and average diameter of minitubers are significant and showed an increase of 48.03%, 35.53%, 49.52% and 24.21% respectively for G. intraradices and Gi. margarita compared to the control.
Figure 3: Aspect of some minitubers harvested under treatment of G. intraradices and Gi. margarita. Appearance of tuberous seedlings (A) Morphology of the rhizome bearing minitubers (B) Different forms of minitubers obtained (C, D, E and F): minitubers (a) rhizomes (b) root (c) Immature (E) mature (F) minitubers with elongated forms (immature scale (d) and mature scale). Minitubers of control (G) G. intraradices (H) Gi. margarita (I).
Table 3: Parameters evaluated on harvested minitubers under treatments of Glomus intraradices and Gigaspora margarita.
Total chlorophyll contents
A significant decrease of chlorophyll content synthesized in the leaves was noticed per treatment and over time (Figure 4). For all the applied treatments, the higher total chlorophyll contents were obtained on D60, either 10.98±2.78 in the control, 15.42±4.28 in G. intraradices and 13.99±4.37 in Gi. margarita. However, this decrease in total chlorophyll content dropped by 51.16% and 50.46% compared to the 49.45% control between D60 and D180.
Figure 4: Total chlorophyll contents (mg.g-1 of FW) in leaves of cocoyam plants under treatments apply.
Discussion
Evaluation of some biochemical parameters
As regards the influence of the mycorrhizal strains used on the synthesis of certain metabolites in the leaves and roots of cocoyam, their contents were higher in plants treated with Gi. margarita compared to that treated with G. intraradices and that of control at 5% level of significance. Overall, in the roots or leaves, the sugar content decreased with time (Figure 5). The contribution of mycorrhiza significantly affects this sugar content during the 120 days of growth. All significant levels are obtained on D60. Mycorrhization led to an increase in sugars synthesis of 36.69% and 59.81% in G. intraradices and Gi. margarita. While at the roots, the increase is 56.92% and 47.89% compared to the control (Figure 5). At day 180 of the harvest, the sugar content is reduced, either less than or equal to 1 mg.g-1 of FW for all treatments in the leaves, while at the level of the roots, it is noted that it is less than 0, 5 mg.g-1 of FW.
Figure 5: Total soluble sugar content in leaves (A) roots (B) cocoyam during induction of minituberization with Glomus intraradices and Gigaspora margarita.
The amino acids and proline contents increased with time (Figure 6). At 5% level of significance, there was a significant difference between the treatments applied and the control. The high contents were obtained in the leaves in the presence of G. intraradices from D60 to D180, either 13.78±0.03 mg.g-1 of FW, 21.34±0.37 mg.g- 1 of FW and 21.47±0.06 mg.g-1 of FW respectively. In the control and Gi. margarita treatment, these significant levels were obtained on D180 therefore 17.72±0.17 mg.g-1 of FW and 19.54±0.07 mg.g-1 of FW. Moreover, in the roots, we noticed a strong increase in the content of amino acids linked to the mycorrhiza (Figure 6). In the presence of Gi. margarita, the results showed an increase of 32.71% on D120 and 45.16% on D180 and 51.69%, 41.87% and 50% between D60 and D180 respectively compared to the control.
Figure 6: Total soluble proteins content in leaves (A) roots (B) cocoyam during induction of minituberization with Glomus intraradices and Gigaspora margarita.
For proline, a progressive and not significant increase at 5% levelof significance in the leaves at all treatments was observed (Figure 7). However, in the roots Gi. margarita was effective to stimulate proline biosynthesis; it was 0.070±0.005 mg.g-1 of FW; 0.056±0.012 mg.g-1 of FW and 0.070±0.012 mg.g-1 of FW respectively between D60 and the D180 (Figure 7). The value of proline is almost double in G. intraradices between D120 and D180, both 0.060±0.002 mg.g-1 of FW and 0.074±0.002 mg.g-1 of FW.
Figure 7: Phenol content in leaves (A) roots (B) cocoyam during induction of minituberization with Glomus intraradices and Gigaspora margarita.
Similarly, the total soluble protein content decreased during time of experiment, regardless of the treatment, whether in roots or leaves (Figure 8). In the leaves, this content was significant and very important in Gi. margarita 2.99±0.09 mg.g-1 of FW; 2.19±0.04 mg.g-1 of FW and 2.75 ± 0.12 mg.g-1 of FW from day 60 to day 180 (Figure 8). While it was 2.37±0.34 mg.g-1 of FW, in G. intraradices. In the roots, a maximum peak of this content was obtained at D120 with Gi. margarita (5.69±0.08 mg.g-1 of FW).
Figure 8: Total amino acids content in leaves (A) roots (B) cocoyam during induction of minituberization with Glomus intraradices and Gigaspora margarita.
Figure 9: Proline content in leaves (A) roots (B) cocoyam during induction of minituberization with Glomus intraradices and Gigaspora margarita.
Discussion
The contribution of mycorrhizal strains of Glomus intraradicesand Gigaspora margarita significantly influenced growth and minituberization in cocoyam. Morphologically, these mycorrhizae had no significant effect on the number of leaves. These results agree with those of which showed that the application of mycorrhizae during the growth of Vigna unguiculata had no effect on the frequency of leaf appearance. In contrast, result of showed that the mean number of leaves during mycorrhization increased significantly over time in Solanum tuberosum in the presence of Glomus intraradices and G. mosseae [41,42]. It was noted that the number of leaves and the average number of roots in cocoyam decrease over time in all treatments. [43], also observed in Dactylis glomerata that the leaf average decreased over time. This observed decrease can be justified by the foliar and root renewal generally observed in plants. As for the root growth, it depends directly on moisture, oxygen, temperature and sugars sent by the leaves. [44] showed that the presence of mycorrhizae in the rhizosphere had the effect of stimulating root growth. However, leaf area and plant size are significant in mycorrhizal plants. Leaf area and plant size are significant in mycorrhizal plants compared to control. The symbiosis in the presence of G. intraradices and Gi. margarita performed significantly stimulated growth in cocoyam. This mycorrhizal symbiosis would have greatly improved the water and mineral nutrition of plants, as the results showed that fresh biomass was much higher than dry biomass, with ratios varying between 2 and 5. Further, stated that the infection of mycorrhiza fungi change the growth and activity of plant roots by the formation of mycelia on the external surface which caused increase in the absorption of nutrients and water [45]. Similarly, the dry biomass increases, the more mycorrhizal dependence increases. Mycorrhizal Dependency (MD) is defined as the degree to which a plant relies upon mycorrhizal condition to produce its maximum growth or yield at a given level of soil fertility [46]. In the present study, MD increased with time in the presence of G. intraradices, but decrease with time in Gi. margarita. Its high value on D180, in G. intraradices and at D120, in Gi. margarita, is considered as the moment of the highest infective activity of the AM fungi. Microscopic analysis of cocoyam root fragments revealed fungal structures such as intracellular hyphae as well as arbuscules. Arbuscules are respectively structures of exchange and reserve of fungal symbiote [47,48]. No structures such as hartig networks have been identified. This suggests an endomycotrophic nature of cocoyam, and confirms the scarcity of ectomycorrhizal structures in arbuscular plants [49,50]. Concerning the mycorrhizogenic capacity following root colonization, the results showed that cocoyam is a mycotropic plant. The analyzes of mycorrhizal frequency, root system mycorrhization intensity, arbuscular content, mycorrhization intensity in mycorrhizal fragments and the abundance of arbuscular in the root system, of each treatment, showed that the strain of G. intraradices was better in the establishment symbiosis (mycorrhizacocoyam). This root colonization decreases with time, and could be explained by the fact that during the growth the plant realizes the root renewal.
Mycorrhization has improved induction the production of minitubers. Moving from 17 minitubers to an almost double value of 37 minitubers with G. intraradices and 32 in Gi. margarita. The minitubers obtained have significant size. Studies have shown that the application of mycorrhizae leads to an increase in production. Results obtained are in agreement with those of, which showed that the presence of mycorrhizae in the substrate would facilitate yam growth and improve the quality and quantity of yam tubers and from, who showed that the application of Glomus intraradices in the field influenced the growth and production of Solanum tuberosum plants [27,51]. Its results also showed that tuber size, tuber yield, tuber yield, and starch yield were higher in inoculated plants than in non-inoculated plants. Furthermore, photosynthesis improvement in plants through mycorrhiza symbiosis is mainly due to the increase in transporting of inorganic elements from soil to plants. Results obtained showed that total chlorophyll content was higher for all mycorrhizal treatments applied compared to the control. Very significant in the presence of G. intraradices, this total chlorophyll content decreases over time. This decrease could be justify by the fact that the cocoyam plants are already preparing to set up the process of accumulation of the reserves which are consequently the result of the photosynthesis, or, due to drought-induced reactive oxygen species production in thylakoids [52]. These results are contrary to those of, who showed that during tuberization in Solanum tuberosum in the presence of Glomus intraradices and G. mosseae, the chlorophyll content increases over time [42]. Similarly to appreciate the effect of symbiosis on secondary metabolites, some biochemical markers have been demonstrated in this experiment. Results showed that, the total soluble sugar content was significant in leaves and roots in the presence of G. intraradices and Gi. margarita compared to the control. Soluble sugars are the product of photosynthesis. This presence of the important soluble sugars could be justified by the fact that the presence of mycorrhizae in the substrate would have improved the absorption of water and mineral elements in cocoyam, and consequently burster the phenomenon of photosynthesis. This could also explain the significant number of minitubercules obtained in the presence of mycorrhizae. Soluble sugars play a crucial role in the regulation of cell functions [53]. The decrease in sugar content observed over time could be attributed to their participation in the biosynthesis of starch to be stored in tubers. Similar results have been reported in potatoes and during microtuberization in cocoyam by [54,55]. Several physiological and metabolic processes of plant development are regulated in part by the variation of soluble sugars. [56], have shown that the presence of significant content of sugars in the medium induce in the cell, a significant accumulation of either protein or phenols. In results obtained, the decrease in sugar content leads to a decrease in total soluble protein content and phenol content. Plant phenolic compounds are potential candidates as signals during mycorrhizal formation [57]. This phenol content is higher in the leaves than in the roots, this may be justified by the fact that phenolic play important roles in plant development, particularly in lignin and pigment biosynthesis. [58], have shown that the biosynthesis of phenolic compounds in chloroplasts and their accumulation in vacuoles is enhanced by exposure of the plant to light. For proteins, their content is very high in the leaves relative to the roots but decrease over the time. Our results corroborate those of [59], who showed that the total protein content also decreased during the maturation microtubers in the Irish potato. Amino acids are involved in the formation of cells and the biosynthesis of proteins [56]. Results also showed that, contrary to the decreased protein content, the total amino acid content increases significantly over time in the presence of Glomus intraradices and Gigaspora margarita. Amino acids are the most studied biochemical markers in mycorrhizal symbioses in the presence of stress. The amino acid content increased significantly in the leaves and roots between D120 and D180. Amino acids are included in the constitution of many oligopeptides which play the roleof endogenous signals and control the process of organogenesis [60]. Their very high content in leaf is justified by the fact that leaves are the biosynthesis and distribution organs of the amino acids in plants [61]. This increase in amino acid content from D120 coincides with the decrease in total soluble sugar content for all treatments. These results are in agreement with those of [62] who suggested that there is a negative correlation between sugar and amino acid levels in potato tubers. Similarly, this increase in amino acids might contribute to the increase in proline so observed in these results. Proline is an osmolyte key, which help plant to maintain the cell turgor. It appeared to be mainly involved in protection against oxidative stress than osmotic adjustment during initial steps of water stress [63]. Proline content is also higher in the leaves than in the roots. This may be due to the fact that its accumulation normally occurs in cytoplasm where it functions as molecular chaperons stabilizing the structure of proteins and its accumulation buffer cytosolic pH and maintains cell redox status [64].
Conclusion
The objective of this work was to verify the effect of G. intra and Gi. margarita on the improvement of growth and tuberization in cocoyam. The results obtained showed that cocoyam is a mycotrophic plant. Mycorrhization was more significant in the presence of G. intraradices. The DM, showed that day 120, can be considered as the moment of the highest infective activity of the AM fungi. All the mycorrhizal treatment applied has improved photosynthesis at the thirty first day. But that content of chlorophyll decreased with time. Results also showed that, all the different secondary metabolites studied as markers were high in leave then roots. The regulation of sugar content in leave and roots has influence those of proteins and phenols. In the same way, the accumulation of amino acid and proline, highly increased following the contribution of mycorrhiza. This pioneering study, is a first step in improving the production of cocoyam with mycorrhizae.
Acknowledgement
The authors would like to thank the Laboratory of Plant Physiology and Biochemistry, for providing the equipment used in this study and the Laboratory of Biology and Applied Microbiology, IRAD Cameroon, for providing the strains of Glomus intraradices and Gigaspora margarita used.
References
- Smith SE, Read DJ (2008) Mycorrhizal symbiosis, (3rd edn). Academic Press, London, UK, pp. 1-769.
- Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37-77.
- FAO (2012) FAOSTATS-Crops.
- Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Boil Fertil Soil 37: 1-16.
- Marulanda A, Azcon R, Ruiz-Lozano JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119: 526-533.
- Marulanda A, Porcel R, Barea JM, Azcon R (2007) Drought tolerance and antioxidant activities in Lavender plants colonized by native drought-tolerant or drought-sensitive Glomus Species. Microb Ecol 54: 543-552.
- Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163: 1101-1110.
- Estrada-Luna AA, Davies FT (2003) Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J Plant Physiol 160: 1073-1083.
- Ozgonen H, Bicici M, Erkilic A (2001) The effect of salicyclic acid and endomycorrhizal fungus glomus etunicatum plant development of tomatoes and Fusarium wilt caused by Fusarium oxysporum f.sp lycopersici. Turk J Agr For 25: 25-29.
- Alguacil M, Caravaca F, Diaz-Vivancos P, Hernandez JA, Roldan A (2006) Effect of arbuscular mycorrhizae and induced drought stress on antioxidant enzyme and nitrate reductase activities in Juniperus oxycedrus L. grown in a composted sewage sludge-amended semi-arid soil. Plant Soil 279: 209-218.
- Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, et al. (2010) Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 335: 311-323.
- Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 386: 1-19.
- Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2009) Effects of arbuscular mycorrhiza, soil sterilization and soil compaction on wheat (Triticum aestivum L.) nutrients uptake. Soil Tillage Res 104: 48-55.
- Dechassa N, Schenk MK, Claassen N and Steingrobe B (2003) Phosphorus efficiency of cabbage (Brassica oleraceae L. var. capitata), carrot (Daucus carota L.) and potato (Solanum tuberosum L.). Plant Soil 250: 215-224.
- Perez EE, Gutierrez ME, De Delahaye EP, Tover J, Lares M (2007) Production and characterization of Xanthosoma sagitifolium and Colocasia esculenta flours. J Food Sci 72: S367- 372.
- Okoye BC, Agwu Ekwe (2007) Technical efficiency of small holder cocoyam farmers in Anambra State, Nigeria: Implications for agricultural extension policy. Int J Agri Rural Dev 9: 1-6.
- Huang CC, Chen WC, Wang CCR (2007) Comparison of Taiwan paddy- and upland- cultivated taro (Colocasia esculenta L.) cultivars for nutritive values. Food Chem 102: 250-256.
- Chandrasekara A, Josheph Kumar T (2016) Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int J Food Sci Vol. 2016.
- Reyes Castro G, Nyman M, Ronnberg-Wastljung AC (2005) Agronomic performance of three cocoyam (Xanthosoma violaceum Schott) genotypes grown in Nicaragua. Euphytica 142: 265-272.
- Ngouo LV (1988) Contribution to the study of the macabo (Xanthosoma sagittifolium) of Cameroon: Identification and analysis of some obstacles to the production of hybrids. Thesis of Doctorate 3rd cycle, University of Yaounde I, pp. 128.
- Omokolo ND, Boudjeko T, Tsafack T (2003) In vitro tuberization of Xanthosoma sagittifolium (L.) Schott: Effects of phytohormones, sucrose, nitrogen and photoperiod. Sci Hort 98: 337-345.
- Tsafack T, Charles G, Omokolo N and Branchard M (2009) Effect of photoperiod and thermoperiod on microtuberization and carbohydrates levels in cocoyam (Xanthosoma sagittifolium L.Schott). Plant Cell Tissue Organ Cult 96: 151-159.
- Djeuani AC, Mbouobda HD, Niemenak N, Fotso, Elian Hubert, et al. (2014) Effect of carbon source on minituberization of cocoyam (Xanthosoma sagittifolium): Analysis of soluble sugars and amino acids contents. Curr Res Microbiol Biotechnol 2: 519-526.
- Niemira BA, Saï¬r GR, Hammerschmidt R, Bird GW (1994) Production of prenuclear minitubers of potato with peat-based arbuscular mycorrhizal fungal inoculum. Agron J 87: 942-946.
- Duffy EM, Cassells AC (2000) The effect of inoculation of potato (Solanum tuberosum L.) microplants with arbuscular mycorrhizal fungi on tuber yield and tuber size distribution. Appl Soil Ecol 15: 137-144.
- Ceballos I, Ruiz M, Fernandez C, Pena R, Rodrıguez A, et al (2013) The in vitro mass-produced model mycorrhizal fungus, Rhizophagus irregularis, significantly increases yields of the globally important food security crop cassava. PLoS One Vol. 8.
- Lu FC, Lee CY, Wang CL (2015) The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. Peer J Vol. 3.
- Raei Y, Weisany W (2013) Arbuscular mycorrhizal fungi associated with some aromatic and medicinal plants. Bull Env Pharmacol Life Sci 2: 129-138.
- Zubek S, Stojakowska A, Anielska T, Turnau K (2010) Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza 20: 497-504.
- Ndoumou DO, Tsala GN, Kanmegne G, Balange AP (1995) In vitro induction of multiple shoots, plant regeneration and tuberization from shoot tips of cocoyam. CR Acad Sci 318: 773-778.
- Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 158-161.
- Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64: 5004-5007.
- Gianinazzi-Pearson V, Gianinazzi S (1985) Physiological and genetical aspects of mycorrhizae. Proceedings of the 1st European symposium on mycorrhizae, Dijon. pp. 832.
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1-15.
- Babu S, Shareef M, Shetty P, Shetty T (2002) HPLC method for amino acids profile in biological fluids and inborn metabolic disorders of aminoacidopathies. Indian J Clin Biochem 17: 7-26.
- Monneveux P, Nemmar M (1986) Contribution to the study of resistance to drought in wheat (Triticum aestivum L.) and in durum wheat (Triticum durum desf.): Study of the accumulation of proline during the development cycle. Agron 6: 583-590.
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteins binding. Anal Biochem 2: 248-254.
- El Hadrami I (1995) Somatic embryogenesis in Phoenix dactylifera L.: Some limiting factors and biochemical markers. Doctoral thesis, State Cadi Ayyad University, Faculty of Sciences-Semlalia, Marrakech, pp. 227.
- Macheix JJ, Fleuriet A, Billot J (1990) Fruit Phenolics. CRC press, Inc. Boca Ranton, Florida, pp. 1-361.
- Singleton VL, Rossi JA (1965) Colorimeter of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144-158.
- Diallo TA (1998) Contribution to the taxonomic and ecological study of Glomales and the influence of mycorrhization with Glomus mosseae and Glomus versiforme on the growth and productivity of cowpea, Vigna ungutculata (L.) Walp. grown under conditions of water deficit. Doctoral thesis of the 3rd Cycle of Plant Biology, Cheikh Anta diop University, Faculty of Science and Technology, Senegal.
- Lone R, Shuab R, Sharma V, Kumar V, Mir R, et al. (2015) Effect of arbuscular mycorrhizal fungi on growth and development of potato (Solanum tuberosum) Plant. Asian J Crop Sci 7: 233-243.
- Kyriazopoulos AP, Orfanoudakis M, Abraham EM, Parissi ZM, Serafidou N (2014) Effects of arbuscular mycorrhiza fungi on growth characteristics of Dactylis glomerata L. under drought stress conditions. Not Bot Horti Agrobo 42: 132-137.
- Brunner I, Brodbeck S (2001) Response of mycorrhizal Norway spruce seedlings to various nitrogen loads and sources. Environ Pollut 114: 223-233.
- Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159: 89-102.
- Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17: 75-91.
- Abbas, Younes (2014) Microorganisms of the Tetracline rhizosphere: A tool to optimize the assisted regeneration of Tetraclinis articulata Vahl. Master. Doctoral Thesis, Mohammed V University, Faculty of Sciences, Rabat, pp. 177.
- Auge RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 25: 13-24.
- Smith SE, Read DJ (1997) Mycorrhizal symbiosis, (2nd edn). Academic Press, Harcourt Brace and Company Publishers, USA, pp. 605.
- Ismael Y, McCormick S, Hijri M (2013) The arbuscular mycorrhizal fungus, glomus irregular, controls the mycotoxin production of fusarium smbuscinum in pathogenesis of potato. Yaacov Okon edition, Federation of European Microbiological Societies 6: pp. 348.
- Adavi Z, Tadayoun MR (2014) Effect of mycorrhiza application on plant growth and yield in potato production under field conditions. Iranian J Plant Physiol 4: 1087- 1093.
- Ramachandra RA, Chaitanya KV, Vivekanandan M (2004) Drought-induced response of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161: 1189-1202.
- Pago JV, Kortstee AJ, Huijser C, Smeekens SC (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51: 407-416.
- Lavieville L, Sangwan BS (1992) Study of the evolution of in vitro microtuberization in Solanum tuberosum (L.) In: from the gene to the company, the first young researcher forum of I.A.P.T.C. (France) pp: 87-91.
- Tsafack TJ (2010) Icrotuberization in Xanthosoma sagittifolium L. Schott and analysis of some physiololic and biochemical aspects. Doctoral Thesis / PhD. University of Yaoundé I. pp. 150.
- Zouine J, El Hadrami I (2004) Somatic embryogenesis in Phoenix dactilifera L: Effect of exogenous supply of sucrose on proteins, sugars, phenolics and peroxidanse activities during the embryogenic cell suspension culture. Biotech 3: 114-118.
- Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5: 359-368.
- Kefeli VI, Kalevitch MV, Borsari B (2003) Phenolic cycle in plants and environment. J Cell Mol Biol 2: 13-18.
- Borgmann K, Sinha P, Frommer WB (1994) Change in two dimensional protein pattern and in gene expression during the sink- to-source transition of potato tubers. Plant Sci 99: 97-108.
- Aldersheim P, Darwill A (1985) Oligosaccharides. Plant Sci 97: 18-19.
- Noctor G, Novitskaya L, Lea PJ, Foyer CH (2002) Co-ordination of leaf minor amino acid contents in crop species: Significance and interpretation. J Exp Bot 53: 939-945.
- Roessner-Tunali U, Urbanczyk-Wochniak E, Czechowski T, kolbe A, Willmitzer L, et al. (2003) De novo amino acid biosynthesis in potato tubers is regulated by sucrose levels. Plant Physiol 133: 683-692.
- Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, et al. (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122: 747-756.
- Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59: 206-216.