Research article
Screening of Agrobacterium Mediated Transient Transformation of Peanut (Arachis hypogaea)
Kashmery Khan* and Aparna Islam
Department of Mathematics and Natural Sciences, BRAC University, 66 Mohakhali, Dhaka 1212, Bangladesh
Corresponding author: Kashmery Khan, Lecturer, Department of Mathematics and Natural Sciences, BRACUniversity, 66 Mohakhali, Dhaka 1212, Bangladesh, E-mail: kkashmery@yahoo.com
Citation: Khan K, Islam A. Screening of Agrobacterium Mediated Transient Transformation of Peanut (Arachis hypogaea). J Plant Sci Res. 2017;4(2): 168.
Copyright © Khan K, et al. 2017. This is an open access article distributed under the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 4, Issue: 2
Submission: 30/04/2017; Accepted: 17/05/2017; Published: 12/06/2017
Abstract
Peanut is a cash crop having outstanding food values. Therefore, it can be used as a supplementary food for hunger and malnutrition affecting Asian countries like Bangladesh. But peanut yield is substantially reduced for abiotic and biotic stresses. Novel and beneficial genes into peanut need to be introduced through genetic manipulation that would not be available using conventional breeding methods. Thus, Agrobacterium mediated genetic transformation may lead the solution. Present study was examined with two Bangladeshi peanut varieties, such as, BINA Chinabadam 4 and BINA Chinabadam 6 where decapitated half embryo was used as explant for transformation. Agrobacterium tumefaciens strain LBA4404 with plasmid constructs, pBI121 was used for infection in the transformation experiment. The total size of pBI121 is 12.8 kb according to its construction map. The T-DNA of Ti plasmid contains a plant selectable marker gene neomycin phosphotransferase II (npt II) conferring resistance to kanamycin and a uidA gene encoding β-gluduronidase (GUS) reporter gene (1812 bp). Explants having different transformation treatments (different OD600, infection and co-cultivation duration, age of the embryo) were taken for GUS histochemical assay. Transformation study was conducted with 1 day old decapitated half embryo where highest percentage of GUS positive explants were found when OD600 was more than 1 at 60 minutes of infection and 3 days of co-cultivation duration in transient assay.
Introduction
Peanut is an annual herbaceous legume plant. Its kernels are rich in protein (25-28%) and oil (48-50%), and are source of several vitamins, minerals, biologically active polyphenols, flavonoids and isoflavones [1]. Introducing novel and beneficial genes into peanut can be possible through genetic manipulation that would not be available using conventional breeding methods. Attempts of conventional hybridization can be taken by several wild Arachis species having novel genes. But conventional breeding between cultivated peanut and A. paraguariensis have failed, as they are cleistogamous.
Among all the species of Arachis, Arachis hypogaea is the only species that has been domesticated as a manner that can be grown worldwide. Established tissue culture protocols have been used for the transformation of peanut which has found in many previous reports [2-7]. There are two major purposes for which tissue culture has been carried out, such as, production of large number of plantlets and propagation of the selected genotypes without inducing any genetic variation [8].
There are two common methods available which were applied for gene delivery, named, Agrobacterium tumefaciens mediated gene transfer and particle bombardment method using gene gun. It has been earlier reported that higher rate of transformation (4.5%) was achieved using gene gun in zygotic embryo of peanut compared to only 1.8% with Agrobacterium tumefaciens mediated gene transfer [2]. However, Agro-based transformation is considered as a cleaner approach, since the T-DNA which is thought to be the only DNA which is transferred into the plant’s genome [9]. Moreover, wider availability and cost effectiveness are also two facilities which can be achieved through this methodology of gene transfer [10].
The Agrobacterium-mediated transformation technique is extensively affected by various factors at different stages of experiment. After agroinfection, shoot regeneration pattern from cotyledon or other meristematic explants was observed rapid and efficient in a number of legume species [11]. Decapitated half embryo is one of those types of explant. Conversely, transformation efficiency was found better when work was done by leaf tissue as explants [12]. Moreover, regeneration and transformation frequency were explants wise analyzed among three types of explants namely leaflet, hypocotyl and epicotyl of peanut and found better regeneration in leaflet [7]. However, the use of decapitated half embryo as explants for transformation in peanut has not been reported yet whereas it is one of the finest explants for transformation of lentils, another .recalcitrant crop [13].
It is necessary to evaluate factors involves in gene transfer prior to taking any transformation project just after the identification of compatible explants. Factors affecting transformation in chickpea were optimized by Akbulut and his colleagues [14]. Islam and her coworkers stated the effects of optical density at 600 nm, incubation period for infection and co-cultivation period in three Bangladeshi tomato varieties together with one Indian tomato variety [15]. Earlier, comparable study was reported by Sharma et al. where they optimized factors, like, bacterial density and co-cultivation time in three Indian varieties of tomato [16].
To develop a robust and reproducible protocol of Agrobacteriummediatedtransformation using best responding explants, determination of the optimum level of transformation factors, such as, genotypes, age of embryo, bacterial density, infection duration and co-cultivation duration is the prerequisite when transformed with the vector, named, pBI121.
Materials and Methods
Matured seeds of three different varieties of peanut, named, BINA Chinabadam 4 and BINA Chinabadam 6 were used for transformation study. These were collected from Bangladesh Institute of Nuclear Agriculture (BINA). Seeds were preserved at 4 °C temperature in Plant Biotechnology Laboratory, BRAC University, Mohakhali, Dhaka, Bangladesh. Agrobacterium tumefaciens strain LBA4404 with plasmid constructs, pBI121 was used for infection in the transformation experiment. The total size of pBI121 is 12.8 kb according to its construction map. The T-DNA of Ti plasmid contains a plant selectable marker gene neomycin phosphotransferase II (npt II) conferring resistance to kanamycin and a uidA gene encoding β-gluduronidase (GUS) reporter gene (1812 bp). These two genes were separately fused under the control of the Nopaline Synthase Promoter (NOS-pro) and CaMV 35S promoter (CaMV 35S-pro) within the left and right border region. For regeneration initiation, decapitated half embryo was cultured on MS media supplemented with different concentrations and combinations of various growth regulators, such as, BAP and Kn. After shoot initiation same concentration of hormone containing MS media were used for shoot elongation. For induction of root from the in vitro grown multiple shoots, half strength of MS basal medium supplemented with IBA was used.
Two state of YEP (Yeast Extract Peptone Broth) with appropriate concentrations of antibiotics were used for bacterial culture. Liquid YEP medium was used for growing Agrobacterium tumefaciens strain LBA4404. This bacterial suspension was used as working culture for infection. Agar solidified YEP medium were used for maintenance of bacterial pure culture. 10 mg of X-Gluc (β-glucuronide, cyclohexylammonium salt, C14H13BrCINO7 C6H13N), (1 mg/ml) was dissolve in 100 μl of Dimethyl Formamide (DMF) in a Pyrex tube. Volume was made upto 10 ml with 50 mM phosphate buffer, pH 7.0. X-Gluc solution was stored in dark container at -20 °C.
Tissue segments were immersed in fixation solution in sterile eppendorf tubes and incubated for overnight. Then the solution was discarded and washed the tissue three times with 50 mM phosphate buffer, pH 7.0. Enough X-Gluc solution was added to cover the tissue pieces in eppendorf tubes. Incubated at 37 °C overnight and allow the blue color to develop. X-Gluc solution was discarded and ice cold 70% ethanol was added and again incubated at 37 °C for 48 hours for degreening. Slides of transformed explants were prepared for observing under microscope.
Optical Density at 600 nm (OD600) of the overnight grown culture was measured while comparing with autoclaved fresh liquid YEP media by using spectrophotometer. The Petri-dish with filter paper is soaked with liquid MS media and then the Petri-dish was used to cut explants. Explants were dipped in bacterial suspension for 30, 60, 90 and 120 minutes for infection and then placed on co-cultivation medium and kept there for next 1 to 3 days (co-cultivation period). The Petri-plates were checked for bacterial overgrowth. Some of the explants having different transformation treatments (Different OD600, Infection and Co-cultivation duration, Age of the embryo) were taken for GUS histochemical assay.
Results
In the present study, LBA4404 Agrobacterium strain harboring pBI121 binary vector was used for the infection of decapitated embryo explants of three peanut varieties. Following infection, explants were analyzed by GUS histochemical assay to understand various factors..on transformation (Figures 1 and 2).
.
Figure 1: Observation of GUS positive explants. A. Blue coloration due to GUS histochemical assay observed in the decapitated half embryo, B. Bacterial overgrowth due to high bacterial density in the co-cultivation medium and GUS positive explants under stereomicroscope (Olympus, Japan) C. BINA Chinabadam 4 and D. BINA Chinabadam 6.
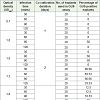
Maximum percentages of GUS positive explants were observed at OD600 of 1.5 and at OD600 of 1.2 in the varieties BINA Chinabadam 6 and BINA Chinabadam 4 (Tables 1 and 2), respectively. For both the test varieties, BINA chinabadam 4 and BINA chinabadam 6, maximum percentage of GUS positive explants was observed after 60 minutes of infection. Further increase of infection duration caused decrease in the percentage of GUS positive explants for both the varieties. It was also seen that with the increase of infection duration from 90 to 120 minutes the percentage of GUS positive explants remained constant at 50% for BINA Chinabadam 6 while the percentage dropped to 33% for BINA Chinabadam 4 (Tables 1 and 2). In addition, the explants were allowed to co-cultivate for 1-3 days where 3 days of co-cultivation duration gave the highest percentage of GUS positive explants in both, BINA Chinabadam 6 and BINA Chinabadam 4 (Tables 1 and 2). The result of positive transformation from 1 day of co-cultivation was not satisfactory at all.
Table 1: Analysis of various transformation factors on transformation of decapitated half embryo of BINA Chinabadam 6.
Table 2: Analysis of various transformation factors on transformation of decapitated half embryo of BINA Chinabadam 4.
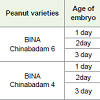
Decapitated half embryo taken from 1 day old seed of BINA Chinabadam 6 and BINA Chinabadam 4 gave 75% and 25% of GUS positive explants respectively which were also the highest percentage obtained when compared to 2 days and 3 days old explants for both the varieties (Table 3). OD600 of 1.0, 60 minutes of infection duration and 3 days of co-cultivation duration were maintained in all the experiments.
Table 3: Effect of age of embryo on transformation efficiency of decapitated half embryo analyzed by transient GUS histochemical assay for BINA Chinabadam 4 and BINA Chinabadam 6.
Discussion
In the current experiment, previously determined optimum hormonal supplementation of Chowdhury was used to evaluate the shoot and root formation potential for three peanut varieties [17].
Decapitated half embryo explants was reported as viable explants for several Bangladeshi varieties, such as, Dhaka Chinabadam 8, BINA Chinabadam 2 and BINA Chinabadam 3. In another effort, presence of embryo axes showed better regeneration capability than cotyledon segments [18].
Decapitated half embryo was found better as explants by Somers et al. [11]. In the present study, peanut varieties, namely, BINA Chinabadam 4 and BINA Chinabadam 6 were with tested with Agrobacterium Strain LBA4404 containing pBI121 (containing nptII marker gene and uidA gene) plasmid vector for determination of influencing factors of genetic transformation for peanut. However, previous studies showed that transformation rate was found to be proportional to the relationship between infected (transformed) explants and inoculation time, co-cultivation time, bacterial concentration and selection antibiotic concentration [19,20].
In the same way, transformation frequency increased with increase of bacterial density and thereafter, decreased with further increase in number of Agrobacterium cells [21]. According to them, higher concentration of Agrobacterium during transformation may cause hypersensitive response of explants as well as it will be a difficult work to kill them after co-cultivation due to excessive aggregation of Agrobacterium cells. Similar result found in Nicotiana tabacum and Arabidopsis thaliana and in most of the grain legumes [22,23]. Infection duration plays a diverged role in transformation. It was seen for Black gram that further increase of infection duration from the optimum value (20-30 minutes) couldn’t help to increase the transformation frequency and caused problems in eliminating the bacteria [21]. This view was also observed by the present study where better transient transformation was found in 60 minutes of infection period in decapitated half embryo. On the other hand, browning of the target tissue had been seen in gherkin due to extending infection time [24].
Conclusion
Co-cultivation duration can be varied on the basis of genotype [25]. In the present experiment, 72 hours co-cultivation time showed the highest percentage of GUS positive explants as the result of ransient transformation in case of decapitated half embryo of BINA Chinabadam 4 and BINA Chinabadam 6. On the other hand, 72 hours was found as optimum duration in another two study where the working explants were cotyledonary node and cotyledon [4,5,16]. Lower co-cultivation duration than the present study was found as sufficient for better transformation in canola whereas higher cocultivation duration such as 4 days is needed for better transformation efficiency in peanut and in alfalfa [26-28].
References
- Janila P, Nigam SN, Pandey MK, Nagesh P, Varshney RK (2013) Groundnutimprovement: Use of genetic and genomic tools. Front Plant Sci 4: pp. 23.
- Higgins CM, Dietzgen RG (2000) Genetic transformation, regeneration and analysis of transgenic peanut. Australian Centre for International Agriculture Research, Cornell University pp. 1-86.
- Sarker RH, Mustafa B, Biswas A, Mahbub S, Nahar M, et al. (2003) In vitro regeneration in lentil (Lens culinaris Medik.). Plant Tissue Cult 13: 155-163.
- Sharma KK, Anjaiah VV (2000) An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens-mediated genetic transformation. Plant Sci 159: 7-19.
- Anuradha TS, Jami SK, Datla RS, Kirti PB (2006) Genetic transformation of peanut (Arachis hypogaea L.) using cotyledonary node as explant and a promoterless gus::nptII fusion gene based vector. J Biosci 31: 235-246.
- Tiwari S, Tuli R (2009) Multiple shoot regeneration in seed-derived immature leaflet explants of peanut (Arachis hypogaea L.). Sci Hortic 121: 223-227.
- Sarker RH, Islam A (1999) In vitro regeneration and genetic transformation of peanut (Arachis hypogaea L). Dhaka University J Biol Sci 8: 1-9.
- Al-Joboury KR (2012) In vitro propagation of groundnut (Arachis hypogea L.). Ibn Al-Haitham J Pure Appl Sci 25: 14-19.
- Smith R, Gould J, Ulian E (1992) Method for transforming plants via the shoot apex. US Pat pp. 5.
- Rafiq S (2014) Analysis of factors influencing successful Agrobacteriummediated genetic transformation in two different explants of peanut (Arachis hypogaea L.) variety BINA Chinabadam-2. BRAC University, Department of Mathematics and Natural Sciences, Dhaka.
- Somers DA, Samac DA, Olhoft PM (2003) Recent advances in legume transformation. Plant Physiol 131: 892-899.
- Cheng M, Jarret RL, Li Z, Xing A, Demski JW (1996) Production of fertile transgenic peanut (Arachis hypogaea L.) plants using Agrobacterium tumefaciens. Plant Cell Rep 15: 653-657.
- Hassan F, Hoque MI, Kiesecker H, Jacobsen H-J (2007) Transient GUS expression in decapitated lentil embryos. Plant Tissue Cult Biotechol 17: 97- 102.
- Akbulut M, Yucel M, Oktem HA (2008) Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L.) seedlings by Agrobacterium tumefaciens. Afr J Biotechnol 7: 1011-1017.
- Islam A, Chowdhury J, Seraj ZI (2010) Establishment of optimal conditions for an Agrobacterium-mediated transformation in four tomato (Lycopersicon esculentum Mill.) varieties grown in Bangladesh. J Bangladesh Academy of Sci 34: 171-179.
- Sharma MK, Solanke AU, Jani D, Singh Y, Sharma AK (2009) A simple and efficient Agrobacterium-mediated procedure for transformation of tomato. J Biosci 34: 423-433.
- Chowdhury IM (2014) Analysis of factors influencing successful Agrobacterium-mediated genetic transformation in two Bangladeshi peanut (Arachis hypogaea L.) varieties BINA Chinabadam-2 and BINA Chinabadam-6 using embryonic leaflet explant. BRAC University, Department of Mathematics and Natural Sciences, Dhaka.
- Vasil IK (1986) Cell culture and somatic cell genetics of plants: Plant regeneration and genetic variability, (Volume 3). Academic Press, Inc, Florida.
- Padmanabhan P, Sahi SV (2009) Genetic transformation and regeneration of Sesbania drummondii using cotyledonary nodes. Plant Cell Rep 28: 31-40.
- Zia M, Rizvi ZF, Rehman RU, Chaudhary MF (2010) Agrobacterium mediated transformation of soybean (Glycine max L.): Some conditions standardization. Pak J Bot 4: 2269-2279.
- Saini R, Jaiwal PK (2007) Agrobacterium tumefaciens-mediatedtransformation of blackgram: An assessment of factors influencing theefficiency of uidA gene transfer. Biol Plant 51: 69-74.
- Lin J, Garcia-Assad N, Kuo J (1994) Effect of Agrobacterium cell concentration on the transformation efficiency of tobacco and Arabidopsis thaliana. Focus 16: 72-77.
- Bean SJ, Gooding PS, Mullincaux PM, Davies DR (1997) A simple system for pea transformation. Plant Cell Rep 16: 513-519.
- De Clercq J, Zambre M, Van Montagu M, Dillen W, Angenon G (2002) An optimized Agrobacterium- mediated transformation procedure for Phaseolus acutifolius A. Gray. Plant Cell Rep 21: 333-340.
- Zhang Z, Coyne DP, Mitra A (1997) Factors affecting Agrobacterium-mediated transformation of common bean. J Amer Soc Hortic Sci 122: 300-305.
- Opabode JT (2006) Agrobacterium-mediated transformation of plants: Emerging factors that influence efficiency. Biotechnol Mol Biol Rev 1: 12-20.
- Venkatachalam P, Geetha N, Jayabalan N, Saravanababu, Sita L (1998) Agrobacterium-mediated genetic transformation of groundnut (Arachis hypogaea L,): An assessment of factors affecting regeneration of transgenic plants. J Plant Res 111: 565-572.
- Chabaud M, Passiatore JE, Cannon F, Buchanan-Wollaston V (1988) Parameters affecting the frequency of kanamycin resistant alfalfa obtained by Agrobacterium tumefaciens mediated transformation. Plant Cell Rep 7: 512-516.