Review Article
Putatively Expressed Proteins in GM Crops for Insect Pest Resistance
Shobhika Parmar* and Vir Singh
Corresponding author: Gulamnabi L Vanti, Lectins and Glycobiology laboratory, Department of Biochemistry, KarnatakUniversity, Dharwad, 580003, India, Phone: +919986545508, Fax: +91836274884; E-mail: nabisam1@gmail.com
1Lectins and Glycobiology laboratory, Department of Biochemistry, Karnatak University, Dharwad, 580003, India
Citation: Vanti GL, Swamy BM. Putatively Expressed Proteins in GM Crops for Insect Pest Resistance. J Plant Sci Res. 2015;2(2): 136.
Copyright © 2015 Vanti GL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 2, Issue: 2
Submission: 01/10/2015; Accepted: 30/10/2015; Published: 07/11/2015
Abstract
The growing demands to produce more food and fibre to feed increasing population and also for reducing chemical inputs in agriculture to overcome adverse affects impetus to the development of alternative forms of insect-pest control strategies. Biological entomotoxic molecules provide an attractive alternative candidate to the use of synthetic pesticides. These molecules are naturally occurring in the organisms and proved to be less toxic to non target pests.
Keywords: Biological molecules; Transgenic plants; Insect pests
Introduction
Challenges facing global agriculture
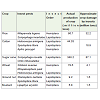
Agriculture is an essential component of societal well-being andit occupies 40% of the land surface, consumes 70% of global waterresources and manages biodiversity at genetic, species andecosystem levels. According to data provided by World Bank, theworld population is currently about 7 billion on October 31, 2011,increase in the population holds serious implications on global foodsecurity, because of these the available necessary biotic and abiotic(nonliving) production factors are shrinking. Agriculture in the 21stcentury faces multiple challenges; it has to produce more food andfibre to feed a growing population. It is estimated that the planet’sdemand for food and feed crops will almost double by 2050 [1], thiscan be achieved largely through higher yields per unit of land andnovel practices of crop intensification [2]. With an increasing globalpopulation and overall purchasing power, the global per capita foodrequired was 2800 kcal/day [3]. The impact of climate change is likelyto reduce agriculture production, thus reducing food availability [4]and also most studies concluded that climate changes also influencesthe eruptive dynamics of pest insects [5]. Moreover, pest population generally become more abundant as temperature increases, througha number of inter-related processes, including increased rates ofpopulation development, growth, migration and overwintering [6].However agriculture production is greatly affected by biotic stressby variety of pests including insects, nematodes, virus, bacterial andfungal induced diseases and weeds, which resulting in losses as highas 41%. Among these, insect pest menace is the major factor thatdestabilizes crop productivity in agricultural ecosystems by morethan 10,000 species worldwide [7,8]. Amongst different orders ofagriculture insect pests; Dipterans, Lepidopetarn and Homeopteranare major insect pests which cause severe damage to economicallyimportant agricultural crops. Some of the important agriculture pestsin India are presented in Table 1.
Crop loss due to these harmful insect pests can be substantialprevented or reduced by crop protection measures by usingpesticides. Pesticide is any substance or mixture of substances used todestroy, suppress or alter the life cycle of insect pest. A pesticide canbe a synthetically produced (Chemical control agents) or substancenaturally derived (Biological control agents).
Synthetic pesticides are not a modern invention, and it’s proved to be effective and most widely used in the world. Insect pestmanagement by synthetic chemicals obviously has brought aboutconsiderable protection to crop yields over the past five decades. It isestimated that, 5.2 billion pounds of pesticides used worldwide yearlyto control insect’s pest [9]. Chemical pesticides have played a role inincreasing agricultural output throughout the globe. Unfortunately,extensive and, very often, indiscriminate usage of chemical pesticideshas resulted in environmental degradation, adverse effects on humanhealth and other organisms, eradication of beneficial insects anddevelopment of pesticide-resistant insects. Alternatives to syntheticpesticides, use of biological pest controls strategies by employingmolecular biology techniques and genetic engineering during thepast few decades led to the introduction of novel strategies for insectcontrol and pest management. These powerful techniques allowectopic expression of single or multiple endogenous putative defenceproteins that are toxic to crop pests [10,11]. The aim of the presentstudy is to introduce and highlight insecticidal activity of some theimportant biological molecules from different sources.
Role of Transgenic plants in agriculture
A sub-branch of plant biotechnology, Transgenic technology, aimsto transfer genes from one species to another either related or not. Theprocess of introducing a gene into an organism via recombinant DNAtechnology is known as transformation and recovered plant speciesare called as transgenic plants or genetically modified (GM) plants.Genetically engineered crops offers user-friendly, environmentfriendlyand consumer-friendly method of crop development to meetthe demands of sustainable agriculture in the 21st century. Transgeniccrops offers the prospect of many advantages; not just widening thepotential pool of useful genes but also permitting the introduction ofa number of different desirable genes at a single event and of reducingthe time needed to introgress introduced characters into an elitegenetic background [12]. Plant biotechnology can potentially helpto higher yields within shorter growing duration, avoiding chemicalfertilizers and increased nutrients quality [13]. As discussed, GM technology enables the development of new crop varieties, whichhave beneficial characteristics for farming; this could be resistance todrought, pests or diseases.
Principle and ectopic expression of entomotoxicbiological molecules
Bt protein (Bacillus thuringiensis)
Bacillus thuringiensis (Bt), a well-known gram-positive bacterium,produces δ-endotoxins, or insecticidal crystal proteins (Cry proteins)during the sporulation phase [14]. The crystals, upon ingestion by theinsect larva, are get solubilized in the alkaline midgut into individualprotoxins. These protoxins are acted upon by midgut proteaseswhich cleave them into two halves, the N-terminal half is responsiblefor the production of the toxic Bt protein. This crystal protein wasexploited in the plant transgenic research to overcome crop damageby insect pests. The Belgian company Plant Genetic Systems was thefirst company to develop a genetically engineered plant with insecttolerance by expressing a cry gene from Bt in tobacco in 1987. Thetobacco plants engineered with cry1Aa genes and the cry1Ab toxinswere found to be resistant to the larvae of Manduca sexta (tobaccohornworm) [15]. Subsequently, many crop plants which includecotton, rice, maize, peanut, soybean, canola, tomato, potato andcabbage were transformed with various modified cry genes [16]. Thecommercialization of Bt-crops started in 1996 with the introductionof bollworm-resistant cotton (‘Bollgard’) in USA. Introduction ofBt endotoxin genes into crops became immensely popular, and it isestimated that Bt crops are cultivated in 66 m hectares area worldwide[17]. However, these transgenic crops expressing Bt endotoxin havebeen effective in controlling chewing pests but are less effective inproviding protection against sap-sucking pests [18]. Despite thegreat commercial successes of Bt crops, there are growing concernsabout the ability of insect to develop resistance to the Bt endotoxins[17,19, 20]. In contrast to Bt crops, alternative sources of potentialinsecticidal gene products need to be explored. To date, severaldifferent classes of proteins including lectins, ribosome-inactivatingproteins, protease inhibitors and α-amylase inhibitors and manymore have been shown to be insecticidal effects towards a range ofeconomically important insect pests by direct assay or by expressionin transgenic plants [21,22].
Vegetative Bt protein
A novel class of proteins called vegetative insecticidal proteins(Vips) produced by Bt during its vegetative stages of growth have beenidentified [23]. Although B. thuringiensis δ-endotoxins are effectiveinsecticidal proteins, there are several agronomically importantinsects that are less sensitive to their action. The 88 kDa vegetativeinsecticidal protein has a putative bacillar secretory signal at theN-terminal which is not processed during its secretion. Vegetativeinsecticidal proteins (Vip) are effective against lepidopteran insectlarvae black cutworm (Agrotis ipsilon), fall armyworm (Spodopterafrugiperda), beet armyworm (Spodoptera exigua), tobacco budworm(Heliothis virescens), and corn earworm (Helicoverpa zea). However,Vip does not show any homology with the known crystallineinsecticidal proteins. This structural dissimilarity is indicative of apossible divergent insecticidal mechanism than the other known Bt- toxins. These observed structural divergences of Vip with Bt toxinsmake them an ideal candidate for deployment in insect managementprograms together with the other category of Bt-toxins.
Proteinase inhibitors
The protease inhibitor (PIs) proteins are natural antagonist’sproteins and they are quite common in all life forms [24]. In plantsthey play defensive mechanisms against phytophagous insects andmicroorganisms. Most PIs interact with their target proteases bycontact with the active (catalytic) site of the protease, resulting in theformation of a stable protease-inhibitor complex that is incapableof enzymatic activity [25]. Proteinases inhibitors are classifiedaccording to their catalytic mechanisms into four classes (1) serineproteinases, with a serine and histidine; (2) cysteine proteinases,with a cysteine; (3) aspartic proteinases, with an aspartate groupand (4) metalloproteinases, with a metallic ion (Zn+2, Ca+2 or Mn+2)[26]. In plants, proteinase inhibitors have different role, such asstorage proteins, as regulators of endogenous proteolytic activity[27], as participants in many developmental processes, includingprogrammed cell death and as components associated with theresistance of plants against insects and pathogens [28]. They may besynthesized constitutively during normal development or may beinduced in response to insect and pathogen attacks [27].
Transgenic plants expressing PI genes began when Hilder etal. [29] transformed tobacco plants with the trypsin inhibitor gene(CpTI) of Cowpea which showed reduced growth and mortality inlarvae of Heliothis virescens (bollworm). In the 1990s Gatehouse et al.[30] transformed tobacco plants with the trypsin inhibitor gene of soya(Kunitz family) (SBTI), which showed a high inhibitory effect on thelarvae of H. virescens. Many PIs were expressed in transgenic plantswhich conferred the protection against Chrysodeixis eriosoma (greenmeasuring worm), Sesamia inferens (pink stem borer), Spodopteralitura (tobacco budworm), Nilaparvata lugens (brown planthopper),Sitotroga cerealella (Angoumoid grain moth), Tribolium castaneum(brown flower beetle) [30-32]. Considering all the evidence which hasappeared in the text, the effect that the PIs have on insects is evident;however, negative effects have also been shown on beneficial insects[33]. Later it was realized that the insects overcome enzyme inhibitoryproperty (PIs) by altering their enzyme specificity by mutations, thusdampening the hopes of using enzyme inhibitors for transgenic crops[10].
α-Amylase inhibitors
α-Amylases (α-1, 4-glucan-4-glucanohydrolases) are widespreadhydrolytic enzymes found in microorganisms, animals and plants.They catalyze the initial hydrolyses of α-1,4-linked sugar polymers,such as starch and glycogen into shorter oligosaccharides, animportant step towards transforming sugar polymers into single unitsthat can be assimilated by the organism. Higher plants and animalsproduce a large number of different protein inhibitors of α-amylasesin order to regulate the activity of these enzymes [34]. However theseα-AIs are used to generate transgenic plants that are resistant againstinsect pest [35]. The expression of the α-Al gene encoding protein inplant system, such as pea (Pisum sativum L.) and azuki bean (Vignaanguralis L.) showed promising effect against bruchid beetle pests (Coleoptera: Bruchidae) [36]. Rye α-amylase inhibitor expressed intransgenic tobacco seeds (Nicotiana tabacum) caused 74% mortalityin Anthonomus grandis first instar larvae when transgenic seed flourmixture used in artificial diet [37]. However these inhibitors used asplant resistance factors are effective against Coleoptera order andthey cannot be used to control different orders of agriculture insectpests [38-40].
Arcelins
Post harvest loss due to insect pests largely affects the overallfood grain production and consumption and it is estimated to be13%. Arcelins are antinutritional insecticidal seed storage proteins,found in the wild bean Phaseolus vulgaris, which have been shownto prevent infestation by post harvest insect pests such as bruchidbeetles [41]. Amino acid sequence comparison shows that arcelinsbelong to the bean lectin-like family which includes the two typesof phytohemagglutin subunits (PHA-L and PHA-E) and α-amylaseinhibitors [42]. Although the members of this protein family displaysimilar tertiary structures, they differ in their biochemical properties,glycosylation patterns, quaternary structure and sugar bindingspecificities [43]. Insecticidal properties of arcelins variants towardbruchid pests Z. subfasciatus has been reported [44], which is knownto be one of the most important pests of stored beans.
Ribosome-inactivating proteins
Ribosome-inactivating proteins (RIPs) are a group of plantproteins that are capable of specifically and irreversibly inactivatingeukaryotic ribosome’s and inhibits protein translation, which playsan important role in plant defense and hence can be exploited inplant protection [45]. Over a hundred RIPs have been isolated fromvarious plants and bacteria with varying degrees of toxicity. RIPs aresubdivided on the basis of their molecular structure into three distinctgroups. Type I RIPs are monomeric proteins of approximately 30kDa which possess RNA N-glycosidase enzymatic activity. Type IIis a heterodimer consisting of an A-chain with RNA N-glycosidaseactivity associated to one or several B-chain(s) of approximately 35kD, functionally equivalent to the Type I polypeptide [46] linked toa B subunit. The B-subunit is a lectin-like peptide that has strongaffinity for sugar moieties displayed on the surface of cells and helpsto promote translocation through the plasma membrane [47]. WhileType III is synthesized as inactive precursors (Pro RIPs) that requireproteolytic processing events to form an active RIP [48]. Ricin, Abrinand Modeccin are well known examples of RIPs, which irreversiblyinactivate ribosomes by removing a specific adenine from a highlyconserved tetra-nucleotide loop present in the large ribosomalsubunit [49]. Some of these RIPs, such as ricin (type-II RIPs) havehigh toxicity affect against a variety of insects, although these effectsare variable on different insect orders [50]. More recently, Shahidi-Noghabi et al. [51] demonstrated that expression of Sambucus nigeragglutinin (SNA-I, type-II RIPs) from elderberry bark in transgenictobacco has a deleterious effect on two important insect pests, thetobacco aphid Myzus nicotianae and the beet armyworm Spodopteraexigua. Although the biochemical properties of the RIPs are wellstudied, but their exact mechanisms of action at the tissue level ofRIPs-ingested insects are not well understood [51-53]. In view of theseargued apprehensions on the sustainability of Bt crops, and other narrow range of biological pest control molecules, alternative sourcesof potential insecticidal gene products need to be explored whichshows wide range of insect control. Lectins from different sources andclasses are an attractive alternative candidate in transgenic-based pestcontrol strategies [45].
Lectins
Lectins are a class of proteins of non-immune origin that possessat least one non-catalytic domain that specifically and reversibly bindto mono- or oligosaccharide [54]. A typical lectin is multivalent;and because of its specific carbohydrate binding property it is ableto agglutinate cells. Lectins are extensively distributed in natureand several hundred of these molecules have been isolated so farfrom plants, microorganisms, fungi and vertebrates, includingmammals [55]. Because of their unique ability to bind to certainspecific membrane glycoproteins, some of lectin exerts proliferative,antiproliferative, immunomodulatory effects. Such propertiesexhibited by lectins made them useful tools in diverse areas like cancerdiagnosis and therapeutics, virology, structural biology, separationtechnology, bacterial typing and insect toxicology [56,57].
Lectins with insecticidal property
Several biological functions of lectins have been reported [58]among them anti-insect activities have received particular attentionin the pest management strategies [59,60]. Lectins basically bind toglycosylated proteins, as the glycosylation of protein is a key posttranslational event. In organisms there are two types of glycosylationpattern occur such as, N-linked and O-linked based on the linkage ofcarbohydrate moiety to the protein backbone. N-glycans are linked toAsn residues of protein backbone via N-acetyl glucosamine, whereasO-glycans linked to hydroxyl group of serine or threonine residuesvia N-acetyl galactosamine. N- glycans and O- glycans are profuselyfound in insects [61,62], because of lectins unique ability to bind tocertain specific membrane glycoproteins in the insects made themvaluable tool in the pest management science. There are substantialevidences that, lectins bind to gut surface glycans of gut epithelial cellsor bind to secretory gut proteins causing physiological imbalance,resulting in immunomodulatory effect such as apoptosis [60,63,64].However, the molecular mechanism of lectin induced toxicity ininsects has not been understood in detail [60,64].
Many lectins are highly toxic for phytophagous insects; the useof lectins in transgenic plants has yielded positive results on insect’spest belonging to different orders such as Lepidoptera, Coleoptera,Diptera and Hemiptera. Among lectins, Plant originated N-glycanspecific lectins received greater attention due to their toxic effectsagainst broad range of economically important insect pests [62].The first N-glycan specific lectin from plant origin, Galanthus nivalisagglutinin (GNA) has showed toxic effects against hemipterans andother economically important insect pests. GNA has been successfullyengineered into a variety of crops including sugarcane, rice, wheat,potatoes or tobacco to give them a higher resistance against differentorder insect pest. Followed by GNA many plant lectins such as, Wheatgerm agglutinin (WGA), Pisum Sativum Agglutinin (PSA), Phaseolusvulgaris Agglutinin (PHA) and Allium sativum lectin (ASAL) weresuccessfully expressed in important agriculture crops and they have been shown to exert deleterious effects on a range of important pestinsects [60,64]. Unlike the N- linked mannose binding lectins, verylittle is known about O-glycan specific lectin. Recently O-glycan(Gal/GalNAc) specific lectins are reported for their toxic effects oninsect pests. Among plant lectin, Amaranthus caudatus is the onlyreported O-linked glycan specific lectin employed in transgenicplants shown to be effective against insects [65,66]. Compared toplant and animal lectins, very little information is available on lectinsfrom fungal origin [67,68]. In recent years, mushroom and otherfungal lectins of different carbohydrate specificity have got muchattention in agriculture field as a bio-insecticide. All these studiesshows that lectins from different origins, potential to be exploited incrop protection against various insect pests. Thus the use of lectinsfrom different origin have proved to be more efficient ways to controlchewing and sap-sucking insect pests on agriculturally importantcrops.
Conclusion
From the literature available, it has been shown that, the use oftransgenic insect resistant crops reduced chemical pesticides andits secondary effects on living organisms. Thus, the combinationof biological proteins with different modes of action, as well as thecorrect application of IPM has the potential to improve resistanceagainst insects over the long-term. Nevertheless, the potential directand indirect effects of transgenic plants expressing these biologicalmolecules on beneficial insects and higher animal’s needs to beinvestigated comprehensively before it could be used for agriculturalapplication.
References
- Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, et al. (2011) Solutions for a cultivated planet. Nature 478: 337-342.
- Morano M (2013) Global crop production increases three fold over the past 50 years.
- Alexandratos N, Bruinsma J (2012) Food Gap: WRI analysis based on World agriculture towards 2030/2050: The 2012 revision. Rome, FAO.
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, et al. (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319: 607-610.
- Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77: 257-64.
- Cannon RJC (1998) The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Global Change Biology 4: 785-796.
- Dhaliwal GS, Jindal V, Dhawan AK (2010) Insect pest problems and crop losses: Changing trends. Indian J Ecol 37: 1-7.
- Oerke EC (2006) Journal of Agricultural Science. Crop losses to pests 144: 31-43.
- United States Environmental Protection Agency (USEPA) (2011) Pesticide news story: EPA releases report containing latest estimates of pesticide use in the United States. Retrieved September 20, 2012.
- Ranjekar PK, Patankar A, Gupta V, Bhatnagar R, Bentur J, et al. (2003) Genetic engineering of crop plants for insect resistance. Special section: transgenic crops. Curr sci 84: 321-329.
- Miller GT (2004) Sustaining the Earth, 6th edition. Thompson Learning, Inc. Pacific Grove, California. Chapter 9, Pages 211-216.
- Hilder VA, Boulter D (1999) Genetic engineering of crop plants for insect resistance a critical review. Crop Protection 18: 177-191.
- Sharma D (2003) From hunger to hidden hunger. Bio Spectrum 1: 40-41.
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, et al. (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62: 775-806.
- Jouanin L, Bonade-Bottino M, Girard C, Morrot G, Gibaud M (1998) Transgenic plants for insect resistence. Plant Sci 131: 1-11.
- Sanchis V (2011) From microbial sprays to insect-resistant transgenic plants: history of the biospesticide Bacillus thuringiensis. A review. Agron Sustain Dev 31: 217-231.
- Tabashnik BE, Brevault T, Carriere Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31: 510-521.
- Porcar M, Grenier AM, Federici B, Rahbe Y (2009) Effects of Bacillus thuringiensis delta-Endotoxins on the Pea Aphid (Acyrthosiphon pisum). Appl Environ Microbiol 75: 4897-4900.
- Bates SL, Zhao JZ, Roush RT, Shelton AM (2005) Insect resistance management in GM crops: past, present and future. Nat Biotechnol 23: 57-62.
- Tabashnik BE, Gassmann AJ, Crowder DW, Carriere Y (2008) Reply to Field-evolved resistance to Bt toxins. Nat Biotechnol 26: 1074-1076.
- Gatehouse AMR, Gatehouse JA (1998) Identifying proteins with insecticidal activity: use of encoding genes to produce insect-resistant transgenic crops. Pest Sci 52: 165-175.
- Ussuf KK, Laxmi NH, Mitra R (2001) Proteinase inhibitors: Plant-derived genes of insecticidal protein for developing insect-resistant transgenic plants. Curr Sci 80: 847-853.
- Warren GW, Koziel MG, Mullins MA, Nye GJ, Carr B, et al. (1996) Novel pesticidal protein and strains, Patent WO 96/10083, World Intellectual PropertyOrganization.
- Fritz H (2000) Foreword. In K. von der Helm, B.D. Korant, and J.C. Cheronis (eds.), Proteases as Targets for Therapy. Berlin: Springer-Verlag, pp. V-VI.
- Norton G (1991) Proteinase Inhibitors. In J.P.F. D'Mello, C.M. Duffus, and J.H. Duffus (eds.), Toxic Substances in Crop Plants. The Royal Society of Chemistry, pp. 68-106.
- Neurath H (1984) Evolution of proteolytic enzymes. Science 224: 350-357.
- Ryan CA (1990) Proteinase inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28: 425-449.
- Pernas M, López-Solanilla E, Sánchez-Monge R, Salcedo G, RodrÃguez-Palenzuela P (1999) Antifungal activity of a plant cystatin. Mol Plant-Microbe Interact 12: 624-627.
- Hilder VA, Gatehouse AMR, Sheerman S, Barker RF, Boulter D (1987) A novel mechanism for insect resistance engineered into tobacco. Nature 330: 160-163.
- Gatehouse AMR, Shi Y, Powel K, Brough C, Hilder V, et al. (1993) Approaches to insect resistance using transgenic plants. Philosophical transactions of the Royal Society of London Serie B 342: 279-286.
- Oppert B, Morgan TD, Hartzer K, Kramer KJ (2005) Compensatory proteolytic responses to dietary proteinase inhibitors in the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Comp Biochem Physiol C Toxicol Pharmacol 140: 53-58.
- Sagili RR, Pankiw T, Zhu-Salzman K (2005) Effects of soybean trypsin inhibitor on hypopharyngeal gland protein content, total midgut protease activity and survival of the honey bee (Apis mellifera L.). J Insect Physiol 51: 953-957.
- Toledo AL, Severo JB, Jr, Souza RR, Campos ES, Santana JC, et al. (2007) Purification by expanded bed adsorption and characterization of an alpha-amylases FORILASE NTL from A. niger. J Chromatogr B Analyt Technol Biomed Life Sci 846: 51-56.
- Franco OL, Rigden DJ, Melo FR, Bloch CJr, Silva CP, et al. (2000) Activity of wheat alpha-amylase inhibitors towards bruchid alpha-amylases and structural explanation of observed specificities. Eur J Biochem 267: 2166-2173.
- Morton RL, Schroeder HE, Bateman KS, Chrispeels MJ, Armstrong E, Higgins TJ (2000) Bean alpha-amylase inhibitor 1 in transgenic peas (Pisum sativum) provides complete protection from pea weevil (Bruchus pisorum) under field conditions. Proc Natl Acad Sci USA 97: 3820-3825.
- Dias SC, da Silva MCM, Teixeira FR, Figueira ELZ, de Oliveira-Neto OB, et al. (2010) Investigation of insecticidal activity of rye α-amylase inhibitor gene expressed in transgenic tobacco (Nicotiana tabacum) toward cotton boll weevil (Anthonomus grandis). Pestic Biochem Physiol 98: 39-44.
- Ishimoto M, Kitamura K (1989) Growth inhibitory effects of an a-amylase inhibitor from kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: Bruchidae). Appl Entomol Zool 24: 281-286.
- Yamada T, Hattori K, Ishimoto M (2001) Purification and characterization of two alpha-amylase inhibitors from seeds of tepary bean (Phaseolus acutifolius A. Gray). Phytochemistry 58: 59-66.
- Kluh I, Horn M, Hyblova J, Hubert J, Maresova LD, et al. (2005) Inhibitory specificity and insecticidal selectivity of alpha-amylase inhibitor from Phaseolus vulgaris. Phytochemistry 66: 31-39.
- Blair MW, Prieto S, Diaz LM, Buendia HF, Cardona C (2010) Linkage disequilibrium at the APA insecticidal seed protein locus of common bean (Phaseolus vulgaris L.). BMC Plant Biol 10: 79.
- Chrispeels MJ, Raikhel NV (1991) Lectins, lectin genes, and their role in plant defense. Plant Cell 3: 1-9.
- >Mourey L, Pedelacq JD, Birck C, Fabre C, Rouge P, et al. (1998) Crystal structure of the arcelin-1 dimer from Phaseolus vulgaris at 1.9-A resolution. J Biol Chem 273: 12914-22.
- Cardona C, Kornegay J, Posso CE, Morales F, Ramirez H (1990) Comparative value of four arcelin variants in the development of dry bean lines resistant to the Mexican bean weevil. Entomol Exp Appl 56: 197-206.
- Sharma HC, Sharma KK, Crouch JH (2004) Genetic transformation of crop plants for insect resistance: Potential and limitations. Crit Rev Plant Sci 23: 47-72.
- Olsnes S, Pihl A (1973) Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 12: 3121-3126.
- Lord JM, Roberts LM, Robertus JD (1994) Ricin: structure, mode of action, and some current applications. FASEB J 8: 201-208.
- Peumans WJ, Hao Q, Van Damme EJ (2001) Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB J 15: 1493-1506.
- Endo Y, Tsurugi K (1987) RNA N-glycosidase activity of ricin Achain: mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem 262: 8128-8130.
- Wei GQ, Liu RS, Wang Q, Liu WY (2004) Toxicity of two type II ribosome-inactivating proteins (cinnamomin and ricin) to domestic silkworm larvae. Arch Insect Biochem Physiol 57: 160-165.
- Shahidi-Noghabi S, Van Damme EJ, Smagghe G (2008) Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucus nigra) is a determining factor for its insecticidal activity. Phytochemistry 69: 2972-2978.
- Shahidi-Noghabi S, Van Damme EJ, Smagghe G (2009) Expression of Sambucus nigra agglutinin (SNA-I') from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res 18: 249-259.
- Bertholdo-Vargas LR, Martins JN, Bordin D, Salvador M, Schafer, et al. (2009) Type 1 ribosome-inactivating proteins - entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). J Insect Physiol 55: 51-58.
- Lis H, Sharon N (1986) Lectins as molecules and as tools. Annu Rev Biochem 55: 35-67.
- Sharon N (2007) Lectins: carbohydrate-specific reagents and biological recognition molecules. J Biol Chem 282: 2753-2764.
- Swanson MD, Winter HC, Goldstein IJ, Markovitz DMA (2010) A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem 285: 8646-8655.
- Souza MA, Carvalho FC, Ruas LP, Ricci-Azevedo R, Roque-Barreira MC (2013) The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj J 30: 641-657.
- Lam SK, Ng TB (2011) Lectins: production and practical applications. Appl Microbiol Biotechnol 89: 45-55.
- Michiels K, Van Damme EJ, Smagghe G (2010) Plant-insect interactions: what can we learn from plant lectins? Arch Insect Biochem Physiol 73: 193-212.
- Vandenborre G, Smagghe G, Van Damme EJ (2011a) Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72: 1538-1550.
- Lopez M, Tetaert D, Juliant S, Gazon M, Cerutti M, et al. (1999) O-Glycosylation potential of lepidopteran insect cell lines. Biochimica Biophysica Acta 1427: 49-61.
- Vandenborre G, Smagghe G, Ghesquie`re B, Menschaert G, Nagender Rao R, et al. (2011b) Diversity in Protein Glycosylation among Insect Species. PLoS ONE 6: e16682.
- HV, Bhat GG, Inamdar SR, Gudihal RK, Swamy BM (2014) Sclerotium rolfsii lectin exerts insecticidal activity on Spodoptera litura larvae by binding to membrane proteins of midgut epithelial cells and triggering caspase-3-dependent apoptosis. Toxicon 78: 47-57.
- Macedo MR, Oliveira CFR, Oliveira CT (2015) Insecticidal Activity of Plant Lectins and Potential Application in Crop Protection. Molecules 20: 2014-2033.
- Wang Z, Zhang K, Sun X, Tang K, Zhang J (2005) Enhancement of resistance to aphids by introducing the snowdrop lectin gene gna into maize plants. J Biosci 30: 627-638.
- >Wu J, Luo X, Guo X, Xiao J, Tian Y (2006) Transgenic cotton, expressing Amaranthus caudatus agglutinin confers enhanced resistance to aphids. Plant Breed 125: 390-394.
- Guillot J, Konska G (1997) Lectins in higher fungi. Biochem Syst Ecol 25: 203-230.
- Wang HX, Ng TB, Ooi VEC (1998) Lectins from mushrooms. Myco Res 102: 897-906.
- Khan F, Khan MI (2011) Fungal lectins: current molecular and biochemical perspectives. Int J Biol chem 5: 1-20.