Review Article
Phytochemical Constituents and Pharmacological Effects of Dangshen (Codonopsis pilosula), an Important Traditional Chinese Medicine
Li Yani, Wang Yulong, Yin Liming, Han Yaping, Chen Kailing, Liu Fei and Vasudeva Reddy N
Shanxi Zhendong Genuine Regional Drug Development Co., Ltd., Zhendong Science and Technology Park, Guangming South Road, Shangdang District, Changzhi City, Shanxi Province, China
*Corresponding author: Vasudeva Reddy N, Shanxi Zhendong Genuine Regional Drug Development Co., Ltd., Zhendong Science and Technology Park, Guangming South Road, Shangdang District, Changzhi City, Shanxi Province, China. E-mail Id: drreddy0205@qq.com
Copyright: © Yani L, et al. 2025. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information:Submission: 20/04/2025; Accepted: 20/05/2025; Published: 26/05/2025
Abstract
Codonopsis pilosula (Franch.) Nannf. (Dangshen), a vital medicinal herb in Traditional Chinese Medicine (TCM), is renowned for its adaptogenic, immunomodulatory, and multi-pharmacological properties. This review comprehensively examines the phytochemical composition and diverse biological activities of C. pilosula, highlighting its therapeutic potential in modern medicine. The plant contains bioactive compounds, including polysaccharides, alkaloids, triterpenes, polyacetylenes, and phenolic acids, which contribute to its broad-spectrum effects. Pharmacological studies reveal its antioxidant, anticancer, immunomodulatory, antidiabetic, antimicrobial, neuroprotective, hepatoprotective, cardioprotective, and antiviral activities. Key mechanisms include ROS scavenging, Nrf2/Keap1 pathway activation, apoptosis induction via signalling pathway, α-glucosidase inhibition, and immune regulation through MAPK/NF-κB signaling. Notably, C. pilosula polysaccharides (CPPs) demonstrate neuroprotection against Aβ-induced toxicity and enhance cognitive function in Alzheimer’s models, while lobetyolin exhibits selective anticancer effects by disrupting glutamine metabolism. Additionally, C. pilosula
shows promise in metabolic disorders, sepsis management, and myocardial repair. It is noted that C. pilosula compounds exhibited multiple therapeutic effects at low concentrations (IC50 10-50 μg/mL). Despite its therapeutic potential, challenges in sustainable cultivation and quality control necessitate further research. This review underscores C. pilosula’s role as a multi-target herbal medicine, bridging traditional use and scientific validation for future drug development and functional food applications.
Keywords:Codonopsis pilosula; Dangshen; Polysaccharides; Immunomodulation; Neuroprotection; Anticancer; Antioxidant; Traditional Chinese Medicine
Introduction
Codonopsis pilosula (Franch.) Nannf., commonly known as
Dangshen, is a perennial herbaceous plant belonging to the family
Campanulaceae (bellflower family). This medicinally important
species is widely distributed across East Asia, primarily in China,
Korea, and Mongolia. In China, it thrives in temperate regions,
particularly in the provinces of Shanxi, Gansu, Sichuan, and Shaanxi,
where it grows at altitudes ranging from 1,500 to 3,100 meters in
mountainous forests, shrublands, and grassy slopes [1,2].
Botanically, C. pilosula is characterized by its twining stems,
ovate to lanceolate leaves, and bell-shaped greenish-yellow flowers
with purple spots. A well growing C. pilosula plant was represented
in [Figure 1A]. The plant produces tuberous roots [Figure 1B],
which are the primary medicinal part, valued in Traditional Chinese
Medicine (TCM) for their adaptogenic and immunomodulatory
properties. While often referred to as “poor man’s ginseng,” C.
pilosula is taxonomically distinct from Panax ginseng (Araliaceae
family), differing in growth habit (twining vs. erect), chemical
composition (higher polysaccharides but lower ginsenoside content),
and cultivation requirements (hardier and more adaptable to diverse
climates) [3,4].
In TCM theory, it is classified as a premier Qi-tonifying herb,
renowned for strengthening spleen function, nourishing lung Qi,
and enhancing vitality. Though it shares therapeutic overlaps with P.
ginseng (e.g., immunomodulation and energy-boosting effects), C.
pilosula is milder in action, less stimulating, and more suitable for
long-term use, making it a preferred substitute for patients with heat sensitive
constitutions or hypertension [5,6]. Modern pharmacological
studies validate its traditional uses, demonstrating antioxidant,
neuroprotective, and cardioprotective properties attributed to
unique bioactive compounds like lobetyolin (a polyacetylene not
found in Panax species) and codonopsosides (triterpenes structurally
distinct from ginsenosides) [7,8]. Its sustainable cultivation remains
crucial to meet growing demand in pharmaceutical and functional
food industries.
Beyond medicinal applications, C. pilosula is deeply embedded in
Asian culinary traditions. Its roots are incorporated into nourishing
soups, herbal teas, medicinal wines, and congees as both a flavor
enhancer and functional ingredient. The plant’s cultural and economic
significance is reflected in the over 160 approved health products
containing C. pilosula extracts in China alone. Recent research
has expanded its potential applications to include management of
metabolic disorders, neurodegenerative diseases, and as an adjuvant
in cancer therapy [9,10]. However, the rising global demand for C.
pilosula underscores the need for sustainable cultivation, standardized
quality control, and deeper pharmacological validation to ensure its
long-term availability and efficacy. In this review, we comprehensively
examine the phytochemical constituents, pharmacological activities,
and therapeutic mechanisms of C. pilosula, while also addressing
current challenges and future directions for maximizing its clinical
and commercial potential.
Phytoconstituents of C. pilosula:
C. pilosula is a pharmacologically rich herb containing
diverse bioactive compounds that underpin its medicinal value.
Key constituents include alkaloids, triterpenes, polyacetylenes,phenylpropanoids, phenolic acids, flavones, and various other unique
secondary metabolites further broaden its therapeutic potential in
traditional and modern medicine.
Alkaloids of C. pilosula:
C. pilosula produces a diverse array of bioactive alkaloids, which
contribute significantly to its medicinal properties. The structures of
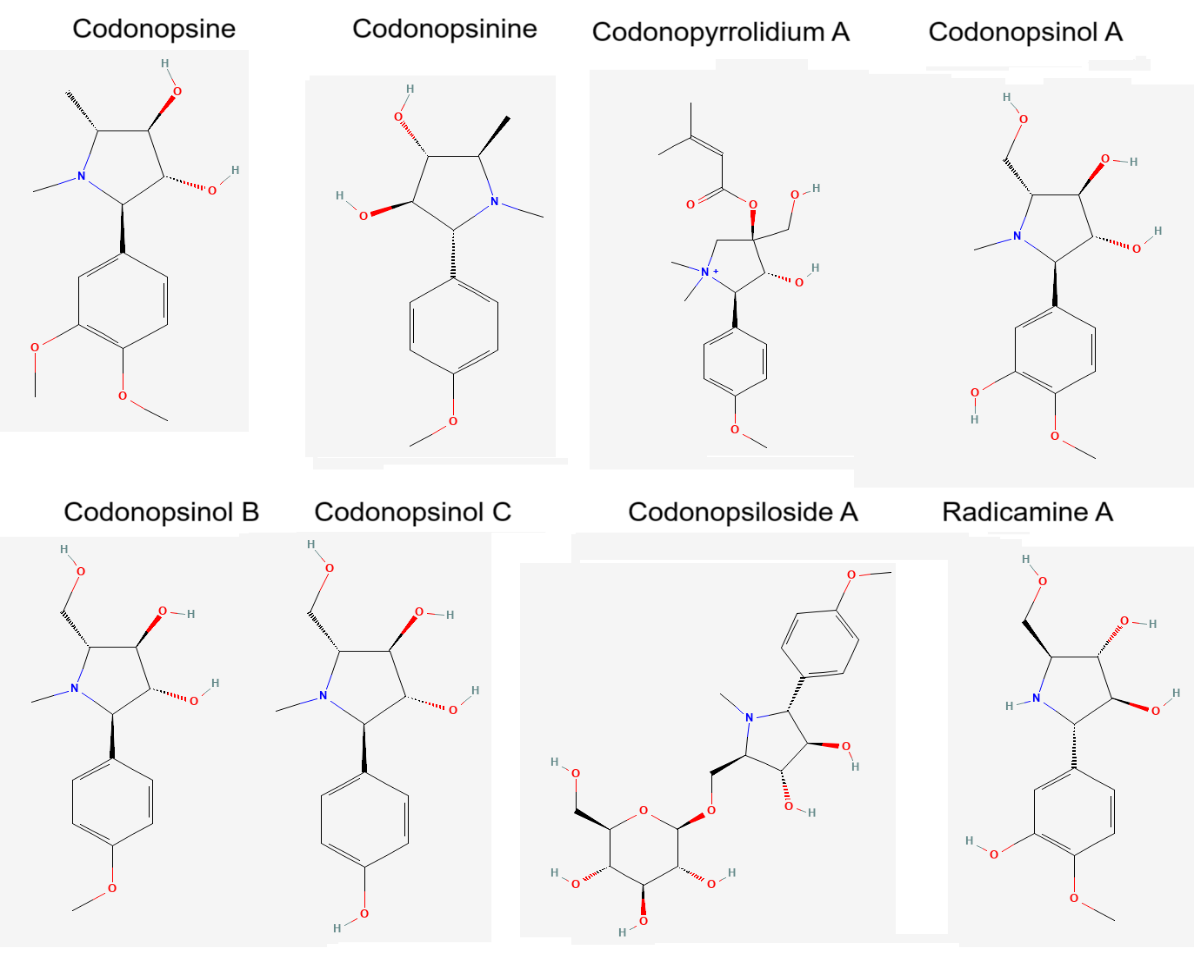
different alkaloids present in C. pilosula were represented in [Figure 2].
Among these, codonopsine and codonopsinine are pyrrolidinetype
alkaloids with potential neuroactive effects. The roots contain
unique pyrrolidine derivatives, including codonopyrrolidium A,B,
D, and E which are considered chemotaxonomic markers for C.
pilosula and its variants (C. pilosulavar. modesta, C. tangshen)[11].
Pyrrolidine alkaloid codonopsinol A, codonopsinol B, codonopsinol
C, codonopyrrolidium B and radicamine A and pyrrolidine alkaloidal
glycoside, codonopiloside A were reported from the roots of C.
pilosula [12].The presence of β-carboline alkaloids like perlolyrine
hints at antioxidant and neuroprotective activities [13], while simpler
nitrogenous compounds such as tryptophan, nicotinic acid (vitamin
B3), and adenosine may contribute to metabolic regulation and
immune modulation. Notably, uracil and adenosine—key nucleosides
in RNA synthesis—could play a role in the plant’s adaptogenic
effects[14,15].Triterpenes of C. pilosula:
The roots of C. pilosula contain a diverse array of triterpenes that
contribute to its medicinal properties.Among these, codonopilates A,
B, C and D are cycloartane-type triterpenes unique to this species,
exhibiting potential anti-inflammatory and hepatoprotective
activities [16,17]. Additionally, Pseudolarolides U and V, two
new triterpenoids were reported from the roots of C. pilosula [18].
The roots also produce pentacyclic triterpenes such as friedelin
and its oxidized form 1-friedelen-3-one, which are associated with
antioxidant and cytotoxic effects. Other notable triterpenes include
stigmast-7-en-3-one and stigmast-7-en-3-ol, which belong to the
stigmas Tane class and may influence membrane stability and
Figure 2:Structures of different alkaloids present in the C. pilosula extracts.
Structures were retrieved from PubChem. Accessed on April 13,2025
signaling pathways. Taraxerol, a lupane-type triterpene found in C.
pilosula has demonstrated anti-tumor and anti-diabetic properties in
preliminary studies. Furthermore, α-spinasterol, a phytosterol with
structural similarities to cholesterol, exhibits anti-inflammatory and
immunomodulatory effects [19]. The presence of taraxeryl acetate,
an acetylated derivative of taraxerol, suggests additional bioactive
potential, possibly enhancing bioavailability. Together, these
triterpenes underscore the pharmacological richness of C. pilosula
roots, with implications for developing natural therapeutics targeting
metabolic disorders, inflammation, and oxidative stress[16-19].
Polyacetylenes of C. pilosula:
The roots of C. pilosula contain three most active polyacetylenes,
a class of compounds characterized by their conjugated acetylene
bonds, which contribute significantly to the plant’s pharmacological
properties. The most prominent polyacetylene in C. pilosula is
lobetyolin, a marker compound often used for quality control
due to its abundance and distinctive bioactivity [20]. Lobetyolin
has demonstrated anti-inflammatory, immunomodulatory, and
potential anticancer effects in preclinical studies. Its structural analog,
lobetyolinin, shares similar properties and may enhance the plant’s
therapeutic profile [21]. Another key polyacetylene, lobetyol, exhibits
cytotoxic activity against certain cancer cell lines, suggesting a role
in antitumor applications [20]. These polyacetylenes are considered
signature compounds of Codonopsis species, and their presence
underscores the plant’s value in traditional and modern medicine
[20,21].Phenylpropanoids, Flavones, and Phenolic Acids in C. pilosula:
The roots of C. pilosula contain a variety of phenylpropanoids,
flavones, and phenolic acids that contribute to its medicinal properties.
Among the phenylpropanoids, syringin (eleutheroside B) stands out
as a key bioactive compound with demonstrated immunomodulatory
and anti-fatigue effects. Another significant group includes the
tangshenosides, particularly tangshenoside I, which shares structural
similarities with ginsenosides and is associated with adaptogenic and
cardioprotective activities. The plant also produces an array of flavones
and phenolic acids, such as luteolin, apigenin glycosides, chlorogenic
acid and caffeic acid derivatives. The presence of hesperidin, a
flavanone glycoside, in the roots adds to its anti-inflammatory and
vascular-protective effects. Together, these compounds underscore
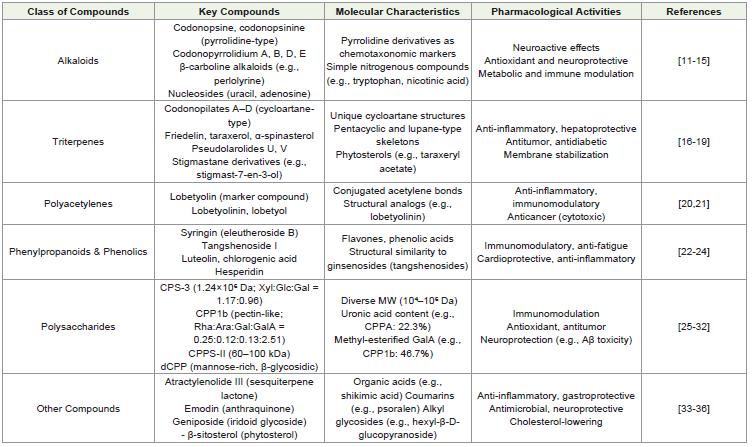
the multifaceted pharmacological profile of C. pilosula [22-24].Polysaccharides of C. pilosula:
C. pilosula contains a diverse array of polysaccharides that vary
in molecular weight, composition, and structural characteristics.
Among these, CPS-3 is a high-molecular-weight polysaccharide
(1.24×10⁶ Da) composed of xylose, glucose, and galactose in
a 1.17:0.96 ratio, while CPS-4 exists as two distinct fractions
with molecular weights of 1.96×10⁶ Da and 1.51×10⁶ Da [25].
Another notable polysaccharide, CPPA, has a medium molecular
weight (4.2×10⁴ Da) and consists of 74.6% carbohydrates and
22.3%uronic acids[26]. The roots also contain CPP1b, a pectin-like
polysaccharide (1.45×10⁵ Da) with a unique monosaccharide profile
(Rha:Ara:Gal:GalA = 0.25:0.12:0.13:2.51) and 46.7% methyl-esterified
galacturonic acid, as well as its selenized derivative, sCPP1b [27,28].
Additionally, CPP1a (1.01×10⁵ Da) exhibits a branched structure
with rhamnose, arabinose, galacturonic acid, galactose, and glucose
in a 1:12:1:10:3 ratio, whereas CPP1c shares similarities but contains
higher uronic acid content[29,30]. Further structural diversity is seen
in 26 CPP variants with varying compositions, along with dCPP, a
mannose-rich polysaccharide (97.2% sugars) featuring β-glycosidic
bonds and a non-helical conformation. CPPS-II represents a 60-
100 kDa fraction, while other unspecified CPP polysaccharides
contribute to the plant’s overall polysaccharide profile. This structural
heterogeneity underscores the biochemical complexity of C. pilosula
polysaccharides [29-32].Other Bioactive Compounds in C. pilosula:
Several organic acids are present, including succinic acid which
participates in energy metabolism, 9,10,13-trihydroxy-(E)-octadec-
11-enoic acid with potential anti-inflammatory effects, and shikimic
acid, a key intermediate in aromatic compound biosynthesis. The
sesquiterpene lactone atractylenolide III exhibits significant antiinflammatory
and gastroprotective activities.The plant also contains
coumarin compounds such as angelicin and psoralen, known for
their phototoxic and potential anticancer effects. Anthraquinones
like emodin contribute to the plant’s laxative and antimicrobial
properties.Additional important constituents include geniposide (an
iridoid glycoside with neuroprotective effects), various alkyl glycosides
(hexyl-β-D-glucopyranoside and butyl-β-D-fructofuranoside) that
may enhance bioavailability, and phytosterols (β-sitosterol and its
glycoside β-daucosterol) which demonstrate cholesterol-lowering
and anti-inflammatory activities[33-36].[Table 1] clearly represents classification of phytoconstituents, key compounds, molecular
characteristics and pharmacological activities of C. pilosula bioactive
components.Biological Activities of C. pilosula:
Antioxidant Properties of C. pilosulaC. pilosula exhibits robust antioxidant activity through a synergistic combination of its diverse phytochemical constituents. The plant’s polysaccharides enhance cellular antioxidant defences by boosting the activity of key enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Flavonoids such as luteolin and apigenin glycosides, along with phenolic acids like chlorogenic and caffeic acids, act as potent free radical scavengers due to their redox-active properties. Additional antioxidant effects come from polyacetylenes (e.g., lobetyolin) and furan derivatives (5-HMF), which neutralize reactive oxygen species (ROS) and chelate metal ions. The β-carboline alkaloids perlolyrine and norharman provide neuroprotective effects against oxidative damage, while triterpenes including taraxerol and α-spinasterol reduce lipid peroxidation by lowering malondialdehyde (MDA) levels[37-40]. Pectic polysaccharides CLRP-1 and CLSP-1 further amplify antioxidant responses by activating antioxidant gene expression. Sulfated polysaccharides (SCP) demonstrate enhanced radical-scavenging capacity in standard antioxidant assays (DPPH, ABTS, FRAP), and leaf extracts show activity comparable to vitamin C due to their high flavonoid and polyphenol content. C.
pilosula polysaccharides (CPPS) exhibit robust antioxidant activity
through multiple mechanisms, including free radical scavenging
and enzyme activation (SOD, CAT, GSH-Px). Two bioactive pectic
polysaccharides (CLRP-1:15.9kDa; CLSP-1:26.4kDa) with distinct
monosaccharide profiles but shared homogalacturonan backbones
demonstrated potent antioxidant effects. Both significantly elevated
SOD, CAT, and total antioxidant capacity while reducing ROS and
MDA in IPEC-J2 cells and C. elegans, potentially via DAF-16 pathway
activation. Their structural features, including arabinogalactan side
chains and high GalA content (66.7-77.0%), correlate with these
protective effects, suggesting their potential as natural antioxidants.
These compounds activate Nrf2/Keap1 and DAF-16 pathways,
upregulating antioxidant genes while reducing ROS/MDA in cells
and C. elegans. The polysaccharides also protect gastrointestinal and
hepatic systems, normalizing ALT/AST and inhibiting oxidative
apoptosis. Together, these compounds work synergistically to provide
comprehensive protection against oxidative stress across multiple
biological systems, supporting C. pilosula’s potential in preventing
and managing oxidative stress-related disorders[40-43].
Anticancer activities of C. pilosula:
Lobetyolin (LBT), a characteristic polyacetylene glycoside from
C. pilosula, exhibits broad-spectrum anticancer activity, particularly
against gastric cancer. Both LBT and its aglycone lobetyol disrupt
glutamine metabolism by downregulating ASCT2 transporter
expression, starving cancer cells of this crucial nutrient and inducing
apoptosis. The compounds demonstrate selective cytotoxicity
against tumor cells while showing minimal effects on normal cells,
suggesting a favorable therapeutic window. Structural analogs like
lobetyolinin (bis-glucosylated form) share similar bioactivity, with
their polyacetylene backbone contributing to membrane interaction
and cellular uptake. LBT’s mechanism extends to modulation of
metabolic pathways and potential interference with oncogenic
signaling cascades.The polyacetylene backbone of lobetyolin (LBT)
and its analogs (lobetyol, lobetyolinin) is critical for membrane
interaction and cellular uptake, while the glycoside moiety (glucose
in LBT) enhances solubility and target specificity. Bis-glucosylation
(lobetyolinin) retains bioactivity but may alter pharmacokinetics,
whereas the aglycone lobetyol shows increased lipophilicity and
cytotoxic potency. The conjugated diyne system in these compounds
is essential for ASCT2 inhibition and metabolic disruption [21].C. pilosula polysaccharide (CPP) demonstrates significant
antitumor activity against NSCLC, showing concentration-dependent
inhibition of A549 cell viability with optimal effects at 40 μmol/L.
The compound induces dual cell death mechanisms - triggering
both apoptosis through ROS accumulation/NF-κB activation and
NLRP3/GSDMD-mediated pyroptosis. In vivo studies confirm
CPP’s tumor-suppressive effects, accompanied by characteristic
pyroptotic morphology and elevated IL-1β/IL-18 levels. The NLRP3
inflammasome-dependent pyroptosis mechanism offers new
therapeutic possibilities for NSCLC treatment[44].CPP demonstrates
contrasting Wnt/β-catenin modulation, promoting proliferation
in hypoxic GES-1 cells (↑Wnt-1/β-catenin/TCF-4) while inhibiting
AGS cancer growth (↓Wnt-1/β-catenin/TCF-4). In PLGC rats, CPP
alleviates gastric damage, improves serum markers, and reverses
weight loss. Western blot analysis revealed CPP upregulates Wnt
pathway proteins in gastric tissue while inducing apoptosis (↓Bcl-
2/Bax ratio, ↑caspase-3). Metabolomics identified CPP’s action on
glycine/serine/threonine metabolism pathways, suggesting multitarget
therapeutic potential against gastric precancerous lesions [45].
C. pilosula aqueous extract (DS) demonstrates significant efficacy
against ulcerative colitis (UC) in TNBS/ethanol-induced rat models,
restoring intestinal barrier function and normalizing oxidative
stress/inflammatory markers. Multi-omics analysis revealed DS
corrects UC-associated metabolic disorders while transcriptomics
identified PI3K/Akt pathway inhibition as its primary mechanism,
downregulating key inflammatory genes. Network pharmacology
pinpointed glycitein as the hub bioactive compound mediating these
effects. DS exhibits dual action by both suppressing pathological
inflammation (via PI3K/Akt blockade) and enhancing antioxidant
defenses, demonstrating concentration-dependent therapeutic
effects[46].Network pharmacology analysis has identified 15 bioactive
compounds in C. pilosula that demonstrate significant potential
against osteosarcoma (OS). The active constituents include sterols
(stigmasterol, stigmast-7-enol, spinasterol, poriferasta-7,22E-dien-
3beta-ol, 5-α-stigmastan-3,6-dione, zinc03978781, taraxerol, and
stigmasterone) inhibits cancer proliferation by modulating steroid
hormone pathways; flavonoids (luteolin, glycitein, and 7-methoxy-
2-methyl isoflavone) regulate apoptosis and DNA damage responses;
alkaloids (11-hydroxyrankinidine and perlolyrine) targeting cell
signalling and migration. These compounds collectively target
48 OS-related genes, influencing critical pathways such as DNA
damage repair, apoptosis induction, cell cycle progression, and
metastasis suppression. Their multi-target action disrupts cancer
cell metabolism, modulates the tumor microenvironment, enhances
chemosensitivity, and regulates immune responses against OS cells
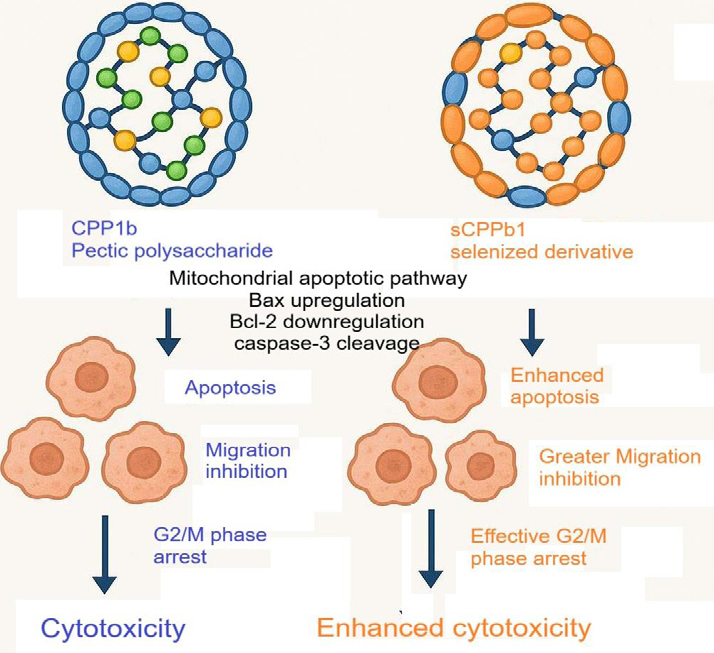
[47].Yang et al. (2013) purified CPP1b, a pectic polysaccharide
derived from C. pilosula, which exhibited concentration- and
duration-dependent cytotoxic effects on A549 non-small cell lung
carcinoma (NSCLC) cells. The observed antineoplastic activity was
attributed to its elevated galacturonate content, with the compound
also demonstrating chemo sensitizing properties when combined
with methotrexate, leading to augmented tumor suppression [27].
In a subsequent study, Chen et al. (2015) synthesized a selenium modified
analog (sCPP1b), which displayed enhanced oncolytic
efficacy across multiple malignant cell lines (A549, BGC-
823 gastric adenocarcinoma, HeLa cervical carcinoma) while
preserving selective cytotoxicity toward non-transformed cells.
The selenylated derivative induced more pronounced pro-apoptotic
effects, stronger anti-migratory activity, and greater G2/M phase
blockade (44.02% vs. 29.81%), along with elevated apoptotic indices
(11.01% vs. 8.14%) compared to the native polysaccharide. Both
compounds triggered mitochondrion-mediated apoptosis via Bax
induction, Bcl-2 suppression, and caspase-3 activation [Figure 3],
with sCPP1b consistently exhibiting superior pharmacodynamic
potency [28].
Immunomodulatory Effects of C. pilosula:
C. pilosula extract (CPE) demonstrated significant
immunomodulatory effects in septic rats, with high doses increasing
thymus and spleen indices while medium/high doses elevated brain
indices. Treatment improved histopathology of these immune organs
and enhanced CD4+ expression, indicating T-cell activation. CPE
restored hematological balance by increasing RBCs, lymphocytes,
and hemoglobin while reducing neutrophils, NLR, and PLR ratios.
It dynamically regulated WBC and platelet counts, along with key
infectious, immune, and inflammatory markers. Metabolomic and
transcriptomic analyses revealed CPE modulates glycerophospholipid
metabolism via the B-cell receptor (BCR) pathway, maintaining
immune homeostasisparticularly humoral immunityin sepsis [48].
The glucan CPC from C. pilosula roots significantly stimulated
RAW 264.7 macrophages, enhancing production of NO, ROS,
iNOS, and cytokines (TNF-α, IL-6, IL-1β, IL-10). It upregulated
mRNA expression of these immune mediators, indicating strong
immunostimulatory effects[49]. C. pilosula oligosaccharides (CPO),
with a 14.3% yield and 92.7% sugar content, consist primarily of
fructose and glucose (DP 1-7, average DP=2). CPO significantly
enhances immune function by stimulating RAW264.7 macrophage
proliferation, phagocytosis, and secretion of TNF-α, NO, and IL-6
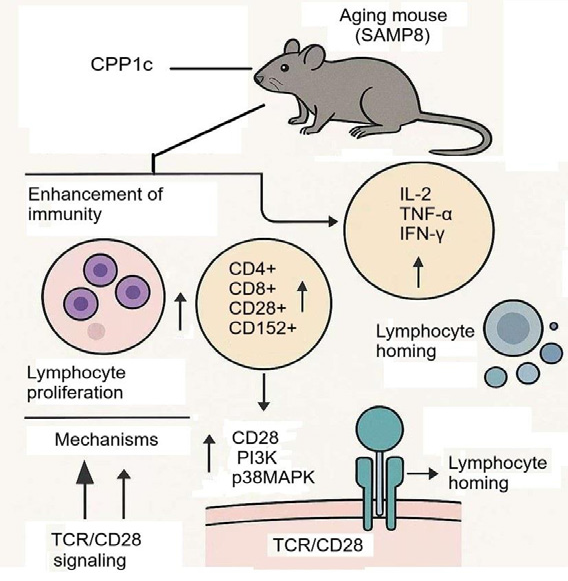
through MAPKs pathway activation[50]. The pectic polysaccharide
CPP1c from C. pilosula exhibits potent immunomodulatory activity
by enhancing T-cell activation through the TCR/CD28 signaling
pathway [Figure 4].In aging mice (SAMP8), it stimulates lymphocyte
proliferation and modulates T-cell subsets, increasing CD4+,
CD8+, CD28+, and CD152+ populations while boosting cytokine
production (IL-2, TNF-α, IFN-γ). Molecular studies confirm that
CPP1c upregulates CD28, PI3K, and p38MAPK at both mRNA
and protein levels, suggesting its role in T-cell co-stimulation and
immune response amplification. Additionally, CPP1c promotes
lymphocyte homing, further supporting its potential as an immune enhancing
therapeutic agent, particularly in aging-related immune
dysfunction [51].Antidiabetic Potential of C. pilosula:
C. pilosula extract (CPNE) demonstrates potent α-glucosidase
Figure 4:Schematic representation of the immunomodulatory effects of
CPP1c, a pectic polysaccharide in aging SAMP8 mice. CPP1c enhances
lymphocyte proliferation by activating TCR/CD28 signaling, upregulating
CD4+, CD8+, and co-stimulatory molecules (CD28+, CD152+), and boosting
Th1 cytokines (IL-2, TNF-α, IFN-γ). Mechanistically, it stimulates the PI3K/
p38MAPK pathway and promotes lymphocyte homing, demonstrating its
potential to counteract age-related immune dysfunction.
inhibitory activity, with IC50 values of 0.241 mg/mL (sucrase), 0.326
mg/mL (maltase), and 1.167 mg/mL (yeast α-glycosidase). In diabetic
mice, CPNE significantly reduces postprandial blood glucose levels
following sucrose/maltose/starch challenges. UHPLC-Triple-TOFMS/
MS analysis identified 29 bioactive compounds, including 3
alkaloids, 13 phenolic acids, 8 alcohol glycosides, and 5 alkynosides,
which likely contribute to its antidiabetic effects. The extract’s dual
inhibition of mammalian and yeast α-glucosidases suggests broadspectrum
carbohydrate-digesting enzyme suppression. These
findings position CPN as a promising functional food or adjuvant
therapy for diabetes management. CPNE’s ability to modulate
postprandial glycemia highlights its potential for preventing
diabetic complications[52].C. pilosula demonstrated significant
plasma glucose-lowering effects in STZ-induced diabetic mice after
4 weeks of treatment. The herb effectively reduced serum aldose
reductase (AR) activity, suggesting potential protection against
diabetic complications. Its antidiabetic mechanism appears linked to
oxidative stress modulation, as evidenced by improved SOD activity
and reduced MDA levels [53].
Six purified polysaccharide fractions (WCP1-6) from C.
pilosula demonstrated distinct bioactivities, with mannose/glucose/
arabinose as primary monosaccharides. WCP3 and WCP5 exhibited
potent inhibition of α-amylase (63.2%) and α-glucosidase (58.7%)
respectively. Molecular docking confirmed WCP5’s stable binding to
digestive enzymes through multiple hydrogen bonds with catalytic
residues. Molecular dynamics simulations (100ns) demonstrated
excellent stability of WCP-enzyme complexes (RMSD < 0.3 nm). The
triple-helix conformation and specific monosaccharide composition
were identified as critical factors for both antioxidant and
hypoglycemic effects[54]. The neutral polysaccharide CERP1 (4.84
kDa), composed of arabinose, glucose, and galactose (1:19.83:6.94),
demonstrated significant antidiabetic potential through its unique
β-linked structure (1,3- and 1,6-glucose; 1,3,6-galactose). In vitro
studies revealed CERP1 enhances insulin secretion in INS-1 cells,
while in T2DM mice it exhibited multi-target effects: reducing
oxidative stress, improving lipid metabolism, and modulating
glycolytic/liver enzymes. The polysaccharide’s homogeneous particle
size and aqueous dispersibility (confirmed by TEM) contribute to its
bioactivity [55].
Antimicrobial activity of C. pilosula:
C. pilosula leaf tea (CLT) and raw leaves (CL) exhibit significant
antimicrobial activity against various bacteria and yeast strains,
while the roots (CR) showed comparatively weaker effects.
The antimicrobial properties are likely attributed to the higher
concentration of bioactive compounds in the leaves, particularly LBT
(0.68 mg/g in CLT vs 0.23 mg/g in CR), flavonoids, and polyphenols.
The aqueous and ethanol extracts of CLT and CL demonstrated
broad-spectrum inhibition, suggesting their potential as natural
preservatives or antimicrobial agents. Interestingly, the tea processing
method enhanced LBT content without compromising antimicrobial
efficacy, making CLT a particularly promising antimicrobial material.
These findings position C. pilosula leaves as valuable alternatives to
the traditionally used roots for antimicrobial applications in food,
cosmetic, and pharmaceutical industries. The dual antioxidantantimicrobial
activity of the leaves further increases their commercial
potential as functional ingredients [39].The orthogonal experiment
identified optimal desulfurization conditions for C. pilosula as 45°C
for 50 minutes with 700W ultrasonic power and a 10:1 ethanol-tomaterial
ratio, achieving a 55.4% desulfurization rate. Desulfurized
polysaccharides demonstrated superior antibacterial activity against
E. coli compared to sulfur-fumigated samples, showing a lower
MIC value (35 mg/mL vs 70 mg/mL). The improved antimicrobial
efficacy suggests that desulfurization effectively preserves bioactive
polysaccharide structures while removing sulfur residues. This process
enhances the therapeutic potential of C. pilosula polysaccharides for
antimicrobial applications [56].Neuroprotective Effects of C. pilosula:
C. pilosula demonstrates significant neuroprotective potential
through multiple bioactive compounds, including polysaccharides,
alkaloids (e.g., codonopsine), and lobetyolin. These components reduce
oxidative stress in neuronal cells by scavenging ROS and enhancing
SOD/GSH-Px activity. The herb modulates neurotransmitter
systems, particularly acetylcholine and dopamine, improving
cognitive function in neurodegenerative models. Its polysaccharides
activate Nrf2/ARE pathways, upregulating endogenous antioxidant
defenses against neurotoxicity. Additionally, C. pilosula inhibits
neuroinflammation by suppressing pro-inflammatory cytokines
(TNF-α, IL-6) and microglial activation. The β-carboline alkaloids
(perlolyrine, norharman) show particular promise in preventing
amyloid-β aggregation and tau phosphorylation. These multi-target
actions support its traditional use for cognitive enhancement and
position it as a potential therapeutic candidate for Alzheimer’s and
Parkinson’s diseases. Further research is needed to elucidate its
blood-brain barrier permeability and clinical efficacy. CPP protects
PC12 cells from Aβ25-35-induced oxidative damage by reducing
ROS/MDA levels, enhancing SOD/GSH/CAT activity, and inhibiting
apoptosis via p38MAPK pathway modulation. The polysaccharide’s
antioxidant and anti-apoptotic effects were reversed by p38MAPK
inhibition (SB203580), confirming this signaling pathway’s critical
role [57]. CPPs significantly improved cognitive function and synapticplasticity (increasing synaptotagmin/PSD95) in APP/PS1 mice while
reducing hippocampal Aβ42/Aβ40 levels. The polysaccharides
inhibited BACE1 activity both in vivo and in vitro, decreasing APPβ
and Aβ42 production. These findings demonstrate CPPs’ dual action
against Aβ pathology through synaptic protection and amyloidogenic
pathway suppression. The BACE1-targeting mechanism positions
CPPs as a promising therapeutic candidate for Alzheimer’s disease
[58]. CPPs improved cognitive function in APP/PS1 mice by reducing
Aβ plaques and hippocampal neuronal apoptosis through modulation
of the PERK-ATF4-CHOP ERS pathway. Treatment downregulated
GRP78, PERK, ATF4, CHOP, and Bax while increasing Bcl-2
expression, demonstrating dual action against amyloidogenesis and
ER stress-induced apoptosis. Molecular docking confirmed CPPs’
affinity for key ERS pathway components, supporting their targeted
mechanism. These findings position CPPs as a promising multi-target
therapeutic for AD by simultaneously addressing protein misfolding
stress and neuronal survival pathways [59]. Hu et al. (2021) reported
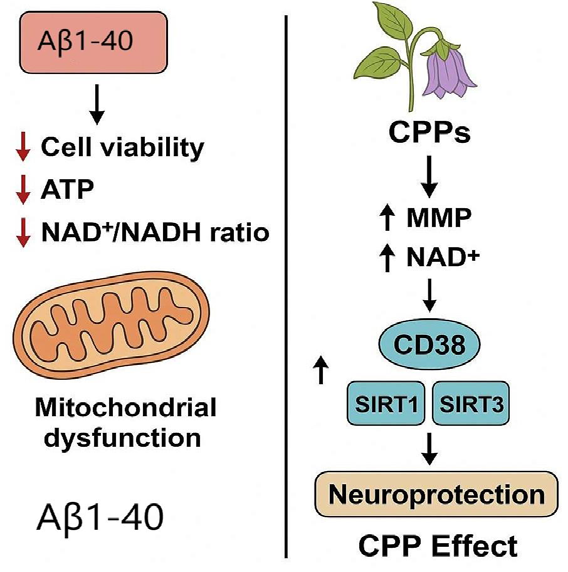
that CPPs exhibit neuroprotective effects in Aβ1-40-exposed PC12
cells, a model for early Alzheimer’s disease (AD). CPPs counteracted
Aβ-induced cytotoxicity, restoring cell viability, ATP production,
and NAD+/NADH balance while mitigating oxidative stress (ROS)
and mitochondrial dysfunction [Figure 5]. Mechanistically, CPPs
enhanced mitochondrial membrane potential (MMP) and preserved
NAD+ levels by suppressing CD38, a key NAD+-consuming enzyme
upregulated by Aβ. This NAD+ preservation activated SIRT1/SIRT3,
critical for mitochondrial homeostasis and antioxidant defense, while
also rescuing PGC-1α expression, a master regulator of mitochondrial
biogenesis. Crucially, CD38 knockdown via siRNA abolished CPPmediated
protection, confirming that their neuroprotective effects
are CD38-dependent. These findings highlight CPPs as a potential
therapeutic strategy for AD by targeting NAD+ metabolism and
mitochondrial function [60].
Hepatoprotective effect of C. pilosula:
C. pilosula exhibits significant hepatoprotective properties through
multiple mechanisms, demonstrating therapeutic potential for liver
disorders. The herb modulates oxidative stress and inflammatory
pathways by upregulating key protective genes including GDF15
and HMOX1, which play crucial roles in cellular repair and redox
balance maintenance. Experimental evidence shows C. pilosula
Figure 5:Neuroprotective effects of CPPs against Aβ1-40-induced toxicity
in PC12 cells, a model for early AD.
enhances hepatocyte proliferation and migration, promoting tissue
regeneration in both hepatocellular carcinoma (HepG2, Huh7) and
normal liver (L-02) cell lines. Its bioactive compounds effectively
reduce liver damage markers and improve hepatic function, as
demonstrated in rat models of liver injury. The herb’s activity aligns
with traditional qi-tonifying properties, counteracting cellular stress
responses characteristic of liver pathologies. The upregulation of
GDF15 indicates potential benefits in angiogenesis and liver tissue
remodeling, while HMOX1 induction underscores potent antioxidant
and anti-inflammatory actions within hepatocytes. The herb’s multitarget
approach to liver protection, addressing both cellular stress and
tissue regeneration, offers a comprehensive strategy for managing liver
disorders while maintaining a favourable safety profile characteristic
of traditional herbal medicines [61].
Cardioprotective Effects of C. pilosula:
The herbal extract 417W from C. pilosula significantly enhanced
cardiogenic differentiation in mouse embryonic stem cells, as
demonstrated by increased α-myosin heavy chain-driven eGFP
expression. In a rat myocardial infarction model, 417W treatment
improved cardiac function for at least 6 weeks post-LAD ligation.
Echocardiography revealed significant enhancements in left
ventricular fractional shortening (FS), fractional area contraction
(FAC), and ejection fraction (EF). These findings validate the
traditional use of C. pilosula for cardiovascular conditions. The
extract demonstrates therapeutic potential for repairing infarcted
myocardium through cardiomyocyte differentiation promotion[62].
Shenqi Fuzheng (SQ) is a renowned traditional Chinese medicine
extracted from Radix Codonopsis and Radix Astragali. Shenqi
Fuzheng (SQ) injection demonstrates multi-target cardioprotection
against ischemia-reperfusion injury by activating PPARα to enhance
myocardial energy metabolism. Network pharmacology revealed its
dual regulation of apoptosis pathways, reducing BAX-mediated cell
death while improving cardiac function. The formulation modulates
inflammatory responses and prevents adverse ventricular remodeling
post-injury[63].Antiviral Activity of C. pilosula:
C. pilosula polysaccharide (CPPS) showed antiviral efficacy
against duck hepatitis A virus (DHAV).Phosphorylation modification
significantly enhanced CPPS’s antiviral efficacy.pCPPS reduced viral
replication (TCID50) and improved survival rates in infected duck
embryonic hepatocytes, unlike unmodified CPPS. The compound
suppressed DHAV-induced IFN-β expression, indicating direct
viral inhibition rather than immune modulation. Structural analysis
confirmed successful phosphorylation, correlating with improved
bioactivity. These findings position CPPS and pCPPS as a promising
antiviral agent for poultry viral hepatitis prevention and treatment
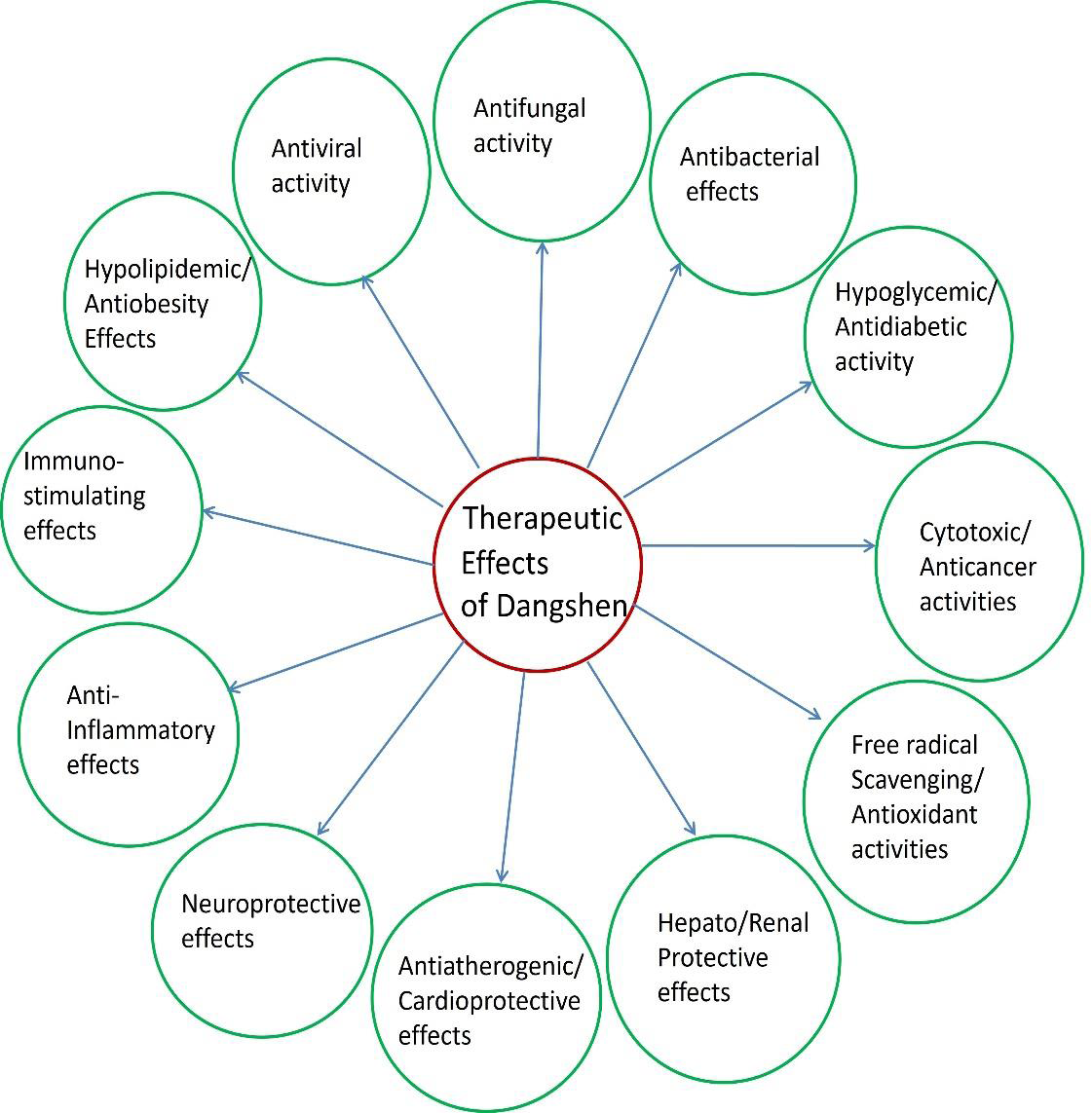
[64]. Multiple therapeutic effects of C. pilosula (Dangshen) were
represented in [Figure 6].Future Perspectives:
Developing standardized cultivation protocols and advanced
analytical methods (e.g., metabolomics, DNA barcoding) to ensure
consistent bioactive compound levels. Addressing challenges like
soil degradation, climate adaptability, and adulteration throughbiotechnological interventions.Elucidating molecular targets and
signaling pathways of key compounds (e.g., CPPs, lobetyolin)
through multi-omics approaches. Advancing preclinical studies to
human trials for diabetes, neurodegenerative diseases, and cancer
therapy.Exploring nano-delivery systems (e.g., polysaccharidebased
nanoparticles) to enhance bioavailability and targeted
action. Developing synergistic herbal combinations or synthetic
analogs to amplify therapeutic efficacy.Integrating C. pilosula into
functional foods (e.g., probiotic synergies, fortified beverages) for
metabolic and immune health. Validating health claims through
clinical studies to meet regulatory standards globally.Promoting
agroecological practices (e.g., intercropping, organic farming) to
reduce environmental impact. Expanding market potential through
value-added products while ensuring fair trade and ethical sourcing.
Conclusions
This comprehensive review systematically examined the
phytochemical composition and multifaceted pharmacological
properties of Codonopsis pilosula, providing scientific validation
for its traditional medicinal applications. The analysis revealed
that key bioactive constituents, especially polysaccharides (CPPs)
and lobetyolin, exhibit remarkable therapeutic effects including
neuroprotection, immune modulation, anticancer activity, and
metabolic regulation through various molecular pathways. The herb
demonstrates significant potential for managing neurodegenerative
conditions, diabetes, cardiovascular diseases, and immune disorders,
attributable to its potent antioxidant, anti-inflammatory, and
cytoprotective capabilities. However, the review also identified critical
challenges that need to be addressed, particularly in standardization
protocols, sustainable cultivation practices, and clinical translation
of research findings. These insights collectively position C. pilosula
as a valuable medicinal resource that warrants further in-depth
investigation to fully realize its potential in pharmaceutical
development and functional food applications, bridging the gap
between traditional herbal medicine and evidence-based therapeutic
use.
Author Contributions:
WY: Writing Manuscript draft, LY: Data curation and Funding
acquisition; HY and YL.: Figures and Data curation; CK and
LF. Tables and References; V.R.N. Validation and Final Version
Correction; All authors have read and agreed to the published version
of the manuscript.Funding:
Key Laboratory of Ecological Planting and Processing of
Authentic Medicinal Materials in Shanxi Province (Grant Number:
202204010931003)Acknowledgments
We are very thankful to Key Laboratory of Ecological Planting
and Processing of Authentic Medicinal Materials in Shanxi Province
(Grant Number: 202204010931003) for financial support.