Research Article
Ethanol Production Potential of Saccharomyces cerevisiae A10, an Indigenous Yeast Strain from Assam
Das M1, Bhattacharya R1, Saha K2, Mandal S3* and Biswas SR1*
1Department of Botany, Visva-Bharati, Santiniketan, Birbhum–731235, West Bengal, India
2Plant Genomics Laboratory, Department of Natural Sciences, Bowie State University, 14000 Jericho Park Road, Bowie, MD 20715-9465, USA
3Laboratory of Molecular Bacteriology, Department of Microbiology, University of Calcutta, 35, Ballygunge Circular Road, Kolkata–700019, India
2Plant Genomics Laboratory, Department of Natural Sciences, Bowie State University, 14000 Jericho Park Road, Bowie, MD 20715-9465, USA
3Laboratory of Molecular Bacteriology, Department of Microbiology, University of Calcutta, 35, Ballygunge Circular Road, Kolkata–700019, India
*Corresponding author:Swadesh Ranjan Biswas, Department of Botany, Visva-Bharati, Santiniketan, Birbhum, West Bengal, India, Email Id: swadeshranjan.biswas@visva-bharati.ac.in
Sukhendu Mandal, Department of Microbiology, University of Calcutta, 35, Ballygunge Circular Road, Kolkata, India, Email Id: sukhendu1@hotmail.com
Sukhendu Mandal, Department of Microbiology, University of Calcutta, 35, Ballygunge Circular Road, Kolkata, India, Email Id: sukhendu1@hotmail.com
Copyright: © Das M, et al. 2025. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information:Submission: 21/03/2025; Accepted: 09/04/2025; Published: 12/04/2025
Abstract
This study investigates the ethanol production potential of the Saccharomyces cerevisiae A10 isolated from the traditional fermented alcoholic beverage from apple juice by native people of Assam, India. The strain A10 was obtained through an enrichment culture in TGE (tryptone, glucose, and yeast
extract) medium, followed by serial dilution, plating, and repeated streaking to establish a pure culture. Identification of the strain was accomplished using morphological assessments and sequence analysis of the D1/D2 regions of the 26 rRNA gene. Sequence analysis and phylogenetic tree construction
confirmed A10 as Saccharomyces cerevisiae, which shared a high level of similarity, with 99.42% identity and 100% coverage with S. cerevisiae NRRL Y-12632. The ethanol production efficiency of this strain was evaluated in batch fermentation using TGE medium. Optimal fermentation conditions, including
temperature, pH, and sugar concentrations, were determined to maximize ethanol yield. Results showed that S. cerevisiae A10 achieved an impressive ethanol yield of 34.5 g/L after 24 hours of fermentation at 32°C with 8% glucose. This promising ethanol yield underscores the potential of this indigenous
yeast strain for large-scale industrial bioethanol production at a low cost and in a shorter time frame and its significance in promoting traditional fermentation practices in Assam. These results provide a foundation for further research into sustainable strategies for bioethanol production.
Introduction
The rising global demand for renewable energy has intensified
interest in bioethanol as a sustainable alternative to fossil fuels. Yeasts
particularly, Saccharomyces cerevisiae play a key role in bioethanol
production due to their potential to efficiently ferment sugars and
starchy crops into ethanol. To harness this potential, it is crucial to
isolate yeast strains from a variety of unexplored sources and conduct
extensive screenings. Various food substrates, including fermented
milk [1], sugarcane molasses [2], traditional fermented foods and
beverages [3-10], homemade fermented cow milk [11], kefir [12],
and fruit wastes [13, 14] have proven to be rich source for isolating
yeast strains to evaluate their ethanol production potential and stress
tolerance, particularly ethanol toxicity. However, the maximum
ethanol yield from yeasts is limited, which requires a large quantity of
substrates and a prolonged time for fermentation [15,16]. Therefore,
to advance sustainable bioethanol production, it is essential to explore
new yeast strains from unexplored geographical regions.
This present study focuses on an indigenous yeast strain,
Saccharomyces cerevisiae A10, which was isolated from traditional
apple juice fermentation in Assam, India. This beverage is prepared
from fermenting apple juice with Bakhar, a starter cake made from
rice dust and various plant root extracts [17], traditionally by local
tribal communities. It contains a consortium of microorganisms [18]
and is widely consumed by the tribes of Assam. S. cerevisiae A10 was
confirmed through both morphological examination and molecular
techniques, specifically analysing the D1/D2 region of the large
ribosomal subunit. The potential of this indigenous yeast strain A10,
in terms of its low-cost ethanol production in a shorter fermentation
time along with its ability to produce bioethanol from various sugars,
is remarkable compared to other yeasts, as reported in this study.
Further optimization and physicochemical characterization using
glucose-rich waste substrates could lead to the development of a
viable strategy for large-scale ethanol production for industrial use.
Materials and methods
Isolation and morphological features of Saccharomyces cerevisiae A10:
The fermented apple juice sample was serially diluted from 10-1
to 10-8 in water and a 50 μL sample from 10−7 and 10−8 dilution was
spread onto TGE agar (tryptone, glucose and yeast extract (HIMedia),
at pH 6.5 [19] The plates were incubated at 32 °C for 48 h.
A few single colonies with distinct morphology were repeatedly
streaked on TGE-agar to obtain the pure culture.Scanning electron microscopy:
For Field Emission-Scanning Electron Microscopy (FE-SEM)
the strain A10 was prepared without chemical fixatives. The strain
grown overnight in a Tryptone-Glucose-Yeast extract medium was
inoculated into a fresh 1% medium and incubated for 30 minutes.
After centrifugation at 4000 rpm for 7 minutes, the supernatant was
discarded, and the pellet was washed with autoclaved distilled water,
repeating this process three times. The cell pellet was resuspended in
10μl of water. A 95 μL aliquot of autoclaved distilled water was mixed
with 5μL of the cell suspension to create a 100μL cell suspension.
For slide preparation, 2-3μL of the suspension was placed on clean
coverslips and dried at 32°C for 30 minutes to avoid desiccation. After
drying, gold plating was applied for 5 to 7 minutes before imaging
with a ZEISS GEMINI SEM 450.Fermentation conditions and ethanol production:
S. cerevisiae A10 was cultured in TGE medium at a temperature
of 32 °C. 4 % of an overnight-grown culture was inoculated into
fresh TGE medium to enhance growth and ethanol production. Two
separate 100 ml Erlenmeyer flasks were used for fermentation, with
20 ml and 100 ml of TGE medium added to allow for varying levels
of air exposure. To determine the colony-forming units (CFU), the
culture was diluted up to 10-8, and aliquots from each dilution were
plated onto TGE-agar plates. Following incubation at 32 °C, the
colonies were counted. Ethanol production during fermentation was
quantified using the Megazyme enzymatic kit (K-ETOH, Megazyme
Inc., Ireland). The flasks were sealed with parafilm, and each
experiment was performed in triplicate.Isolation of Genomic DNA:
Genomic DNA of strain A1 was extracted from a pure culture
using a previously established protocol [20]. Briefly, cells from
an overnight culture grown at 32°C in 5 mL of TGE [19] were
centrifuged at 5000 × g for 10 minutes. The cell pellet was then
lysed in a mixture of 400 μl lysis buffer (1% SDS and 88 mM sodium
acetate), followed by the addition of 400 μl of TE-saturated phenol
(pH 8). The samples were incubated at 65°C for 10 minutes before
being centrifuged at 5000 × g for 7 minutes at 4°C. The aqueous
supernatant was treated with 20 μl of RNase (10 mg/mL) (Genei,
India) for 30 minutes at 37°C, and subsequently incubated with 10
μl of Proteinase K (Genei, India) at 50°C for 1 hour. Following this,
an equal volume of a phenol/chloroform mixture (1:1, 500 μl) was
added. After another centrifugation at 5000 × g for 7 minutes at 4°C,
DNA was precipitated using sodium acetate (3M) and isopropanol,
and was then resuspended in sterile double-distilled water. The DNA
concentration was quantified spectrophotometrically.PCR amplification of D1/D2 region of 26S rRNA gene of S. cerevisiae A10:
The D1 and D2 region of the 26S rRNA gene of A10 was
amplified using PCR with the conserved fungal primer pair
NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4
(5′-GGTCCGTGTTTCAAGACGG-3′) [21]. These primers were
commercially synthesized (Bio-Kart India). The PCR was conducted
under conditions similar to those described previously [20], utilizing
Pfu DNA polymerase (Fermentas, Hanover, MD, USA) in an Applied
Biosystems 2720 thermal cycler. The thermal cycling program
included an initial denaturation step at 95°C for 5 minutes, followed
by 35 cycles of denaturation at 95°C for 1 minute, annealing at 52°C for
1 minute, and extension at 72°C for 2 minutes, concluding with a final
extension at 72°C for 10 minutes. The PCR products were analyzed by
electrophoresis on a 1% (w/v) agarose gel, with a 100-bp DNA ladder
(New England BioLabs Inc.) serving as a molecular marker. The PCR
products were purified using the QIAquick PCR purification kit
(Qiagen, Hilden, Germany) and sequenced commercially (Bio-Kart,
India), using both primers. Sequence comparisons for homology
assessment were conducted using the BLAST program (http://www.
ncbi.nlm.nih.gov/BLAST). The sequence obtained was submitted
to GenBank with the accession SRA: SRR32731888, Bio sample:
SAMN47283387 and Bioproject:PRJNA1215549.Phylogenetic tree:
The sequences of the 26S rRNA D1/D2 regions were analyzed
using BLAST at the NCBI (National Center for Biotechnology
Information) to align them with known 26S rDNA in the GenBank
database, generating percent identity scores to identify the yeast
strain. Phylogenetic trees were then constructed with MEGA version
11.0 using a neighbor-joining algorithm [22] and the Kimura twoparameter
(K2P) distance measure, with Pachysolen tannophilus
NRRLY-2460 selected as the outgroup species. Bootstrap support for
the neighbor-joining tree was evaluated through 1000 replicates, with
bootstrap values indicated at the branch nodes. The bar represents 2
base substitutions per 100 nucleotides.Results
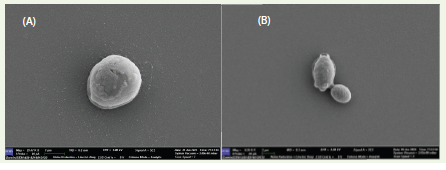
S. cerevisiae A10 was observed under scanning electron
microscope and found to be ovoid with about 3.05 μm in diameter
[Figure 1].
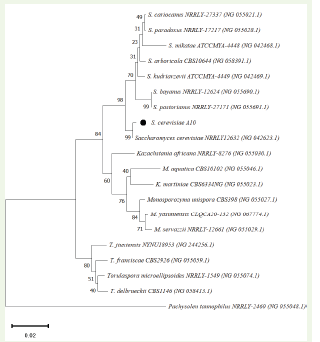
The sequence analysis of the D1/D2 region of the 26S rRNA gene for the strain A10 allowed for species-level identification. A BLAST analysis revealed that the strain exhibited a 99.13% identity with S. cerevisiae NRRL Y-12632. Consequently, isolate A10 was identified as S. cerevisiae and designated S. cerevisiae A10. In the phylogenetic tree, this organism clustered with S. cerevisiae NRRL Y-12632 [Figure 2] further confirming its identity as established by the BLAST analysis.
The sequence analysis of the D1/D2 region of the 26S rRNA gene for the strain A10 allowed for species-level identification. A BLAST analysis revealed that the strain exhibited a 99.13% identity with S. cerevisiae NRRL Y-12632. Consequently, isolate A10 was identified as S. cerevisiae and designated S. cerevisiae A10. In the phylogenetic tree, this organism clustered with S. cerevisiae NRRL Y-12632 [Figure 2] further confirming its identity as established by the BLAST analysis.
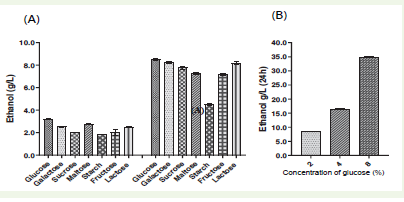
To evaluate the ethanol production potential of S. cerevisiae
A10, seven different sugars-glucose, galactose, sucrose, maltose,
starch, fructose, and lactose (all at 2%)-were assessed. Among these,
glucose emerged as the most effective sugar for stimulating ethanol
production when the yeast was cultured in 20 ml of medium within
a 100 ml flask for 24 hours, the ethanol yield was 3.2 g/L, while the
ethanol yields from the other sugars varied. However, when A10 was
cultured in 100 ml of medium with the same concentration of glucose
in a 100 ml flask, the ethanol production was found to be increased to
8.5 g/L, but still lower than the yields obtained from the other sugars
[Figure 3A]
To evaluate the impact of varying glucose concentrations on
Figure 1:Representation of Field Emission- Scanning Electron Microscopy images captured by ZEISS GEMINI SEM 450, the photograph shows S. cerevisiae A10 single cell morphology (A) with a scale bar of 1μm and budding stage of S. cerevisiae A10 with a scale bar of 2μm.
Figure 2: Phylogenetic tree of S. cerevisiae A10 (accession SAMN47283387). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter = 1). This analysis involved 20 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 542 positions in the final dataset. Evolutionary analyses were conducted in MEGA11
Figure 3:Representation of ethanol produced (g/L). (A) The graph shows the ethanol yielded in 24 hours’ both in aerobic (left) and anaerobic like mimic conditions (right) in seven different types of sugar with a fixed concentration of 2% each. (B) The graph shows the ethanol yielded during fermentation by three different concentrations of glucose (2%,4%,8%) in a fixed time point of 24 hours.
Figure 4:Representation of ethanol production and cell counts (CFU/ml). The graph represents the amount of ethanol (g/L) produced at a concentration of 8% glucose during fermentation at different time intervals (represented in left axis) with the count of cell growth (represented in the right axis) in each time interval.
ethanol production, a range of 2-8% glucose was used in a total volume
of 100 ml medium, set for fermentation in 100 ml Erlenmeyer flasks
with minimal aeration, the fermentation continued for 48 hours. The
highest ethanol production occurred at 24 hours with the 8% glucose
concentration [Figure 3B]. Analysing ethanol production in relation
to growth revealed that S. cerevisiae A10 reached its peak ethanol level
at 24 hours [Figure 4] coinciding with maximum growth, after which
both production and growth remains constant.
Discussion
Traditional fermented beverages have long been valuable sources
for isolating valuable yeast strains, significantly contributing to our
understanding of the microbial world [23]. This study investigates the
unexploited potential of the indigenous yeast strain S. cerevisiae A10
in fermenting sugar. The key to proper ethanol yield always lies on its
better optimization [24]. In this study under optimized parameters
i.e. temperature 32⁰C in anaerobic like mimic condition and a 4%
inoculum size, S. cerevisiae A10 showed noteworthy results.
In a 100 ml flask containing 20 ml of medium, A10 produced an
ethanol yield of 3.2 g/L. However, in an anaerobic-like environment
with nearly no airspace,it achieved an impressive ethanol production
of 8.5 g/L in 100 ml of medium. The flask to volume ratio played an
efficient role in yielding more ethanol in a lesser amount of time.
It is reported that once excessive aeration was avoided the
production was higher [25]. In contrast to other reported yeast
strains, which only produce a maximum of 12% to 15% ethanol [26],
the ethanol yield was impressive in A10 (34.5 g/L). No doubt A10
yielded higher amount of ethanol in 4% and 8% concentrations of
glucose but the major highlight about A10 was its effective amount
of ethanol production even from a low concentration of glucose i.e.
2%. Although the lower concentration of glucose took longer span of
time to yield a high amount of ethanol, but the important part was its
cost- effective value and a higher yield. A10 demonstrates superior
efficacy, highlighting the potential of traditional fermentation
methods for alcohol production. This research not only illustrates
the feasibility of using indigenous yeasts for bioethanol production
but also emphasizes their contribution to sustainable fermentation
practices in their native region.
While ethanol production by yeasts typically peaks at 15%, many researchers have engineered yeast strains to enhance this yield. However, the wild-type S. cerevisiae A10 does not require genetic modification, although further optimization could be beneficial for improving ethanol production outcomes. For industrial applications, establishing low-cost, large-scale production methods using sugary waste materials under optimized conditions is essential [27].
While ethanol production by yeasts typically peaks at 15%, many researchers have engineered yeast strains to enhance this yield. However, the wild-type S. cerevisiae A10 does not require genetic modification, although further optimization could be beneficial for improving ethanol production outcomes. For industrial applications, establishing low-cost, large-scale production methods using sugary waste materials under optimized conditions is essential [27].
Conclusion
In summary, the strain A10 exhibits a substantially improved
ability to produce bioethanol compared to previously studied strains.
This research underscores its promise as a viable renewable energy
source and establishes a foundation for future studies aimed at
sustainable bioethanol production methods. The results indicate that
additional exploration and optimization of this strain could enhance
bioethanol production efficiency, advancing the development of
more environmentally friendly energy solutions.