Research Article
Impact of Commercial Detergents on Organogenesis in Allium Cepa L. and Utility of Foldscope Tool
Pradeep Madhamanchi1, Haritha Ronanki1, Hemanth Paidi1, Kishore Madhamanchi2, M. Abdul Kareem3, Shanthi Devi Chikile4, SPD Ponamgi5, Madhavarao Panchareddi6 and Sujatha Peela6*
1Centre for Applied Sciences, Government Degree College (Men)-Srikakulam, Andhra Pradesh, India
2Department of Biotechnology, School of Life Sciences, University of Hyderabad, Gachibowli, Telangana,India
3Department of Biochemistry, School of Sciences, Academic complex, Indira Gandhi National Open University, Maidan Garhi, New Delhi, India
4Department of Microbiology, Dr. V. S. Krishna Government College (A)-Vishakhapatnam, Andhra Pradesh, India
5Department of Biotechnology, Andra University, Visakhapatnam, India
6Department of Biotechnology, Dr. B. R. Ambedkar University-Srikakulam, Andhra Pradesh, India
2Department of Biotechnology, School of Life Sciences, University of Hyderabad, Gachibowli, Telangana,India
3Department of Biochemistry, School of Sciences, Academic complex, Indira Gandhi National Open University, Maidan Garhi, New Delhi, India
4Department of Microbiology, Dr. V. S. Krishna Government College (A)-Vishakhapatnam, Andhra Pradesh, India
5Department of Biotechnology, Andra University, Visakhapatnam, India
6Department of Biotechnology, Dr. B. R. Ambedkar University-Srikakulam, Andhra Pradesh, India
*Corresponding author: Sujatha Peela, Department of Biotechnology, Dr. B. R. Ambedkar University, Srikakulam, Andhra Pradesh, India. E-mail: drpsujatha@gmail.com
Copyright: © Pradeep Madhamanchi, et al. 2023. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 20/09/2023; Accepted: 10/10/2023; Published: 14/10/2023
Abstract
Detergents are chemically made cleaning agents extensively used in households, industries, hospitals etc. and the spent detergents are released into nearby water bodies. Such detergent polluted water is often utilized for agriculture and home garden irrigation purposes. Much study has not been done
to explore the impact of this polluted water on crop growth and its productivity. The current study tried to demonstrate the impact of some commonly used commercial detergents on crop growth by taking organogenesis in onion (Allium cepa L.) as model system and by utilizing low cost, origami based paper
microscope tool i.e. foldscope. Our study divulged that for most of the detergents the minimum inhibitory concentration of organogenesis was 0.5% and compromised organogenesis was observed at low detergent concentrations (0.006, 0.012 and 0.025%). Stunted roots and decreased root density in onions
were observed at higher detergent concentrations. Altered cell morphology (more dehydrated cells) and disordered cellular arrangement was observed in roots that were grown in high detergent concentrations by using Foldscope.
Keywords: Allium cepa L; Commercial Detergents; Organogenesis; Origami; Foldscope
Introduction
Detergents are cleaning agents, made up of a cocktail of chemical
components and are available as powders or concentrated solutions
[1]. Generally they are sodium salts of long chain alkyl hydrogen
sulphate or a long chain of benzene sulphonic acid [2]. Detergents are
a set of compounds with amphiphilic structures, where each molecule
has a hydrophilic polar head and a long hydrophobic non-polar tail.
The hydrophobic portion of these molecules may be straight or
branched chain of hydrocarbons or it may have a steroid structure.
The hydrophilic portion is more diverse, they may be ionic or nonionic,
and can range from a simple or a relatively elaborate structure
[3]. Due to its amphiphilic and surfactant nature detergents are
extensively used in home cleaning and industrial processes [4].
Moreover, detergents work better at basic or alkaline pH [5]. The
spent detergents and surfactants released from home and industry
enter in to the water bodies causing far-reaching environmental
impacts [6]. Detergents affect the fauna and flora, and they have
direct and indirect effects on ecosystems. Eutrophication, foaming,
and altering parameters such as temperature, salinity, turbidity
and pH are more important and their effects need to be assessed,
managed and controlled [7]. Few reports showed that more than
95% of the commercial detergents can cause damage or disorders
to the respiratory tract, reproductive system, endocrine system and
immune systems [8]. However, no significant reports have been
found on detergents effect on agriculture and its productivity. In this
context to demonstrate the impact of detergents on crop growth and
development we adopted low cost, affordable models: organogenesis
(Roots and shoots development) in onions (Allium cepa L.) and
foldscope tool. Foldscope is an origami based, affordable (~ $1 cost),
simple microscope of 140X magnification that can be assembled from
paper, lens and magnets [9]. The foldscope can connect with mobile
phone, take photographs and videos of the specimen and also could
project image on the screen. It was designed by Dr. Manu Prakash, an
Indian born scientist at Stanford University-USA and aims to make
inexpensive and easy tools available for scientific use in the world,
especially in developing countries [10]. Currently the Department
of Biotechnology (DBT), Ministry of Science and Technology,
Government of India is extensively popularizing this foldscope tool
across the country through workshops and micro grant projects.
Keeping these aspects in view, in the present study we planned to
assess the influence of various commonly used detergents on root
generation and shoot generation in Allium cepa L. Further we sketch
to annexure the impact of detergents on root morphology using
foldscope instrument and also to depict the foldscope potential to use
it as an educational and research tool.
Materials and Methods
The foldscopes used in this study was offered by DBT-New Delhi
under the Indo-US Foldscope project. The powder detergents (Rin,
Ariel, Tide, Ghadi, Surfexel, Sunflower, Wheel, Urvashi & XXX) and
Onions collected from local super market. Saffranin stain and other
lab materials procured from National Scientific Products, Kakinada,
A.P. All the experiments were conducted at Biotechnology Laboratory
of Government Degree College (Men)-Srikakulam, A.P.
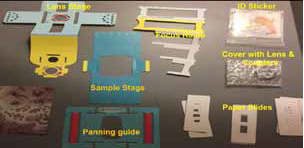
Assembly of Foldscope (AF):
Foldscope is a simple microscope made it from paper (Assembly
sheet), magnets (3-4 no) and a lens (140x). The assembly sheet
comprises of the following major parts- Lens Stage, Sample stage,
Focus ramp and Panning guide (Lhanjey P.W et.al; 2019) [9]. The
details of the assembly process made by our foldscope team given
in this link: https://youtu.be/dgE8GeuLL4w and the photographs of
various parts of foldscope shown in [Figure 1].Determination of Minimum Inhibition Concentration (MIC):
MIC is the minimum concentration of detergent in the solvent
(water) greater than which inhibits the organogenesis in onions. In
order to find the MIC, we prepared a series of detergent concentrations
of 500 ml volume for the selected detergents starting from 10%,
5%, 2.5%, 1%, 0.5%, 0.1%, 0.05% and 0.01%. Each concentration
in triplicates was used to place trimmed (old roots removed from
bulbs) onions and subsequently determined the MIC after 20 days
of incubation.Organogenesis in Onions (OO):
Organogenesis is the process of generation of roots and green
shoot primordium from the onions. After determination of MIC, to
assess OO 1000 ml quantities of 0.1%, 0.05%, 0.025%, 0.012% and
0.006% solutions with Rin detergent powder were prepared. Then, 18
conical flasks of 250 ml volume were divided in to three triplicate
groups each with six flasks. Each triplicate group was marked as 0.1%,
0.05%, 0.025%. 0.012%, 0.006% concentration and a control group.
The control group of flasks was filled with tap water and the other
flasks with the respective detergent solutions. Next 18 comparable
onions were selected carefully dried roots were trimmed and placed
on the top of the conical flask ensuring that the bulb was in contact
with the solution for organogenesis as shown in [Figure 2] Similar
procedure was followed for all the test detergents for organogenesis
in onions [Table 1].Assessment of Root and Shoot Density (ARSD):
ARSD is the process of determining the number of roots and
shoots generated to the experimental onions. The following formula
was used to measure the ASRD.
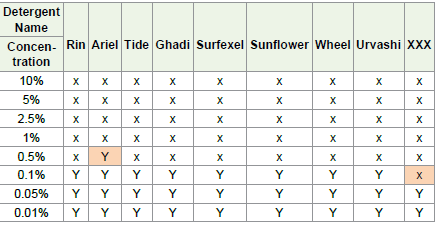
Table 1: Showing MIC for various detergents after 20 Days of incubation: X = No
Organogenesis; Y = Organogenesis
D = N ÷ A
D = Density; N = Number of Roots or Shoots; A = Area of Bulb
or tip of the onion
A =π r2
r = radius of the bulb or tip
π =22/7 or 3.14
D = Density; N = Number of Roots or Shoots; A = Area of Bulb
or tip of the onion
A =π r2
r = radius of the bulb or tip
π =22/7 or 3.14
ARSD was determined after 5 days, 10 days and 20 days of onion
incubation. Every time the onions were taken out from the flask and
carefully dried with tissue paper. Then the onions were placed on a
clean contrast floor or paper shown in [Figure 3] and the roots and
shoots of every onion under study counted manually. Also, the Area
of every onion under study was determined by measuring radius and
the density was calculated. The onions were then replaced in their
respective flasks. Similar procedure was followed for all the test
detergents and organ density calculated.
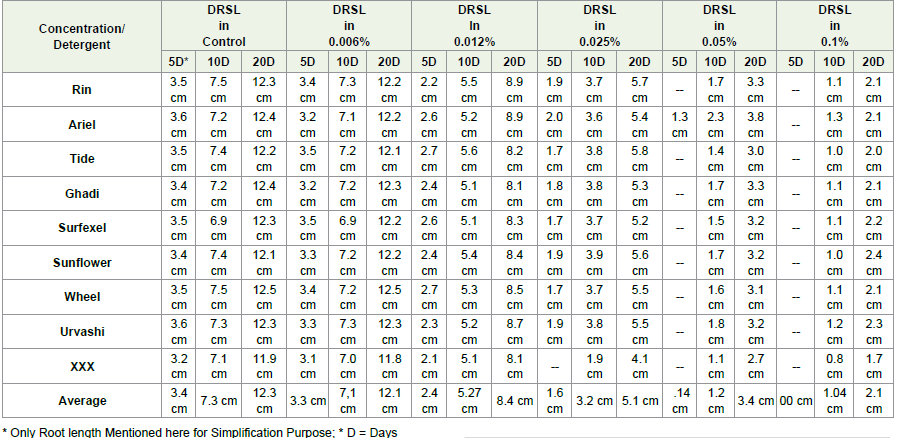
Determination of Root and Shoot Length (DRSL):
DRSL was determined after 5 days, 10 days and 20 days of
incubation. Every time the onions were taken out from the flask and
carefully dried with tissue paper. The onions were then placed on a
clean contrast floor or paper and the extreme lengths (shortest and
longest) of root and shoot were measured using 30 cm scale. Then the
average length was calculated using below formula
A = (S + L) ÷ 2
A = Average Length; S = Length of Shortest Root or Shoot; L =
Length of Longest Root or Shoot
After study the onions were replaced in their respective plasks.
Similar procedure followed for all the test detergents and the root and
shoot lengths were calculated.Study of Root Anatomy (SRA):
To study the influence of detergents on root anatomy, transverse
sections (TS) of all the experimental onion roots were prepared,
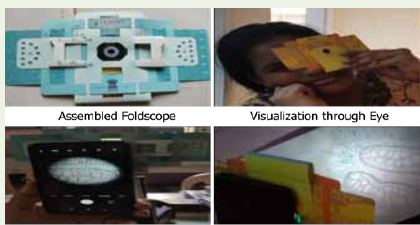
stained, mounted on slides and observed under foldscope. To do
that the onion was removed from flask, the roots were dried carefully
with blotting paper. Then a root was cut from the onion and held in
between the index finger and thumb. Using sharp razor the root was
sliced into thin sections, transferred into water containing watch glass
using a brush. Later 2-4 good root TS were transferred into another
watch glass with Saffranin stain and allowed to set for 5 minutes.
Then the sections were transferred to the watch glass with waterand set to rest for 2 minutes. One of the sections was placed in the
middle of the slide. A drop of glycerin was added on the section and
covered with a coverslip. Excess glycerin was wiped from the edges
of the coverslip with a tissue paper and ensured that no air bubbles
were formed in the mount. The mounted slide was inserted into the
foldscope, adjusted and attached to the mobile and photographs of
the sections were taken. Similar procedure was followed for all the
other experimental onions.
Data Analysis:
All the experiments were repeated independently in triplicates
and all the calculations and graphs relative to control were done using
Microsoft Excel 2007 software.Results
Assembled foldscope and its usage methods shown in the fig-4.
The MIC for all the selected detergents was considered after 20 days
of incubation. The MIC for Rin, Tide, Ghadi, Surfexel, Sunflower,
Wheel, Urvashi detergents was 0.1% in water and for Ariel & XXX it
was 0.5% and 0.05% respectively as shown in [Table 1].
Organogenesis in onions under all the testing detergents was done
after 5 days, 10 days and 20 days of onion incubation. After 5 days of
incubation roots development was observed only at 0.006%, 0.012%
and 0.025% detergent groups along with control. In Ariel detergent
roots were observed in control, 0.006%, 0.012%, 0.025% and 0.05%.
However in XXX roots were found only in control, 0.006% and 0.012%
concentrations. After 10 days of incubation roots were observed in all
the concentration groups while green shoots were observed only in
control and 0.006% groups. After 20 Days of incubation, roots and
shoots were observed in all the groups with significant variations as
shown in the [Figure 3].
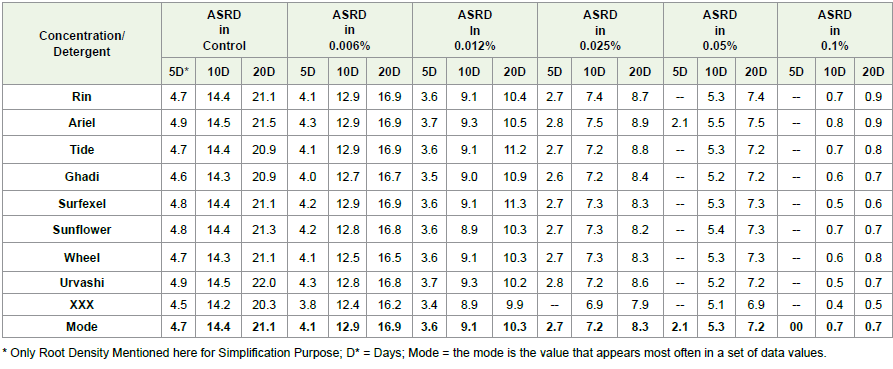
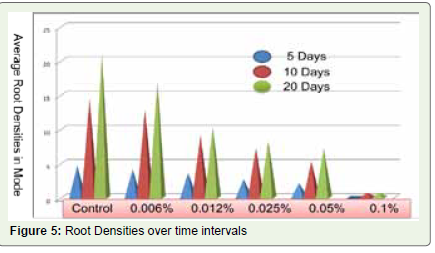
ARSD against time interval was calculated by using the given
formula and average organ densities (Root densities only) for
each detergent concentration and control shown in [Table 2,3]
Considerable improvement in the root density after 05, 10 and 20
days was observed only in control and at low detergent concentration
group i.e. 0.006%. For 0.012%, 0.025% and 0.05% concentrations,
notable improvement in root density was observed in between 05 to
10 days as shown in [Figure 4,5].
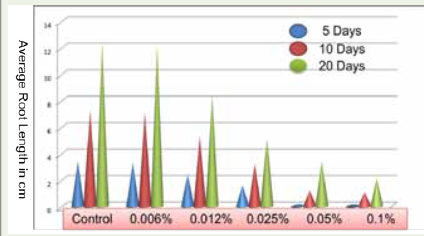
DRSL against time interval was calculated as per given formula
Table 2: Average root densities after 5, 10 and 20 days under each detergent concentration and control
and average organ lengths (Root lengths only) for each detergent
concentration & control shown in [Table 3] Significant improvement
in root lengths after 05, 10 and 20 days was observed in control and
at low detergent concentration group i.e. 0.006% but no growth was
observed after five days in 0.025% and 0.05% concentrations as shown
in [Figure 6].
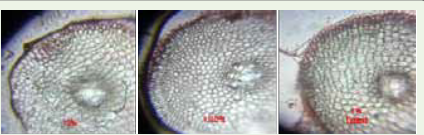
TS of Control roots possessed evenly arranged barrel shaped
epidermal cells with numerous root hairs and its cortex contained
loosely arranged parenchymal cells with prominent intracellular
spaces. Whereas in the TS of roots generated in the detergents
possessed irregularly arranged epidermal cells with no root hairs.
Also the cortex contained compactly arranged heterogeneous cells.
The endodermis of the roots generated in the detergents possessed
thicker casparian bands than that of the control root endodermis.
Enlarged pith region was observed in roots generated in detergents in
comparison to the control roots [Figure 6,7].
Discussion
Detergents are inevitable chemical substances in day to day
human life, but populace has least concern on their disposal,
associated health hazards and negative impacts on environment
especially in agriculture [7]. Very few studies described the impact
of various types of detergents on agriculture and its productivity
also with conflicted conclusions. Some studies described that
detergents at low concentration promote crop growth and at high
concentrations inhibit [11,12]. Other studies showed that detergents
usually promote crop growth and productivity by increasing water
and nutrients absorption of roots [13]. But in our study we used
variety of regular detergents to study its influence on crop growth by
taking organogenesis in onions as study model. For each detergent,
we used a range of concentrations starting with minimum of 0.006%,
surprisingly all tested detergents have negative impact on the
organogenesis as compared with the control. The detergents showed
negative impact on the root and shoot density, their length and also
in their anatomy and morphology. In this study we also found that
detergents significantly affect organogenesis in later days than early
days shown in [Figure 5]. With the study it can be presumed that
detergents may have influence on the plant growth regulators and
suppress the organogenesis. The study also highlighted the significance
of considering the detergent effect while developing high yield crop
varieties. The uniqueness of the study is the usage of low cost, origami
based paper microscope i.e. foldscope for anatomical studies of onion
roots grown in detergents, emphasized the educational and research
potential of foldscope.
Acknowledgements
The authors are grateful to DBT-New Delhi and Manu Prakash
Lab at Stanford University, USA for financial assistance and providing
foldscope. Also grateful to Dr. M. Babu Rao, Principal, GDC M
Srikakulam and Dr. Ch. Tulasi, AGO, CCE-AP, Amaravati for their
moral and administrative support.
Author’s Contribution:
Conceptualization and designing of the research work (PM);
Execution of field/lab experiments and data collection (HP); Analysis
of data and interpretation, preparation of manuscript (KM, HR, SDC
and MAK).
* PM = Pradeep Madhamanchi; HP = Hemanth Paidi; KM =
Kishore Madhamanchi; HR = Haritha Ronanki; SDC = Shanthi Devi
Chikile; MAK = M. Abdul Kareem