Metabolic Engineering of Lipids in Plants
Aditya Banerjee and Aryadeep Roychoudhury*
Corresponding author: Aryadeep Roychoudhury, Post Graduate Department of Biotechnology, St. Xavier’s College(Autonomous), 30, Mother Teresa Sarani, Kolkata - 700016, West Bengal, India,; E-mail: aryadeep.rc@gmail.comaryadeep19@rediffmail.com
Post Graduate Department of Biotechnology, St. Xavier’s College (Autonomous), 30, Mother Teresa Sarani, Kolkata - 700016,West Bengal, India
Citation: Banerjee A, Roychoudhury A. Metabolic Engineering of Lipids in Plants. J Plant Sci Res. 2014;1(3): 112.
Copyright © 2014 Aryadeep Roychoudhury et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 1, Issue: 3
Submission: 17/09/2014; Accepted: 11/11/2014; Published: 12/11/2014
Abstract
Metabolic engineering is the culmination of chemical engineering, computational sciences, biochemistry and molecular biology which aids in the ambitious alteration of metabolic pathways. Such purposeful genetic modifications are required for further understandingand fruitful utilisation of the several biosynthetic pathways. Lipid is an important biomolecule with varied uses in the industrial sector as well as in the human diet chart. In the industrial sector, lipids are required in huge supplies for terpenes, paints, lubricants, octene,sandalwood oil, waxes, hydrocarbon-rich biofuel, biodiesel and bioplastics. The oils found in fishes, like the very long chain fatty acids (eicosapentaenoic acid, docosahexaenoic acid etc.) are essential dietary components. The petroselinic acid and peppermint oil also have potential usages as raw materials for the manufacturing of end products in the market. Targeting of transcription factors in the biosynthetic pathways to enhance lipid accumulation is now being designed in transgenic plants. Metabolic engineering of these lipids in transgenic plant systems allows higher accumulation of target products in the plant tissues. This leads to the overproduction of essential components in a highly cost-effective manner. Such manufacturing processes are adopted to pursue sustainable development and green chemistry, circumventing the ethical issues and environmental hazards. In this review, we present a detailed analysis of the gradual as well as themost recent advances in metabolic engineering of plant lipids in tandem with the several cross-talks present in the biosynthetic pathways of lipid production, along with the adaptive evolutionary patterns in seed oils.
Introduction
Particular lipid biosynthetic pathways with fixed end product fluxes are not always sufficient for industrial or dietary fulfilments. Theidentification of such pathways and isolation of the enzyme coding genes to overexpress them not at the cost of other pathways, in order to derive a genetically modified product with desired phenotype, is called metabolic engineering. The designing of genetically modifiedtransgenic plants has boosted the opportunities to produce industrially useful fatty acids (FAs) in oilseed crops. A transgenic plant generally consists of a heterologous polynucleotide sequence usually attained by genetic engineering. This has been posed as the central role ofgreen chemistry within the context of lipid metabolic engineering in plants. Scientists have identified several genes encoding suitable FA-modifying enzymes from available wild species. Still the major limiting factor in the improvement in this field has been the lowyields of the desired products in plants. Major constituents of plant oils are unsaturated FAs. Corn oil contains 86% unsaturated FAs and 14% saturated FAs. A recent experiment produced a meagre 17% of hydroxyl fatty acids (HFAs), when the Ricinus communis fatty acidhydroxylase 12 (FAH12) was expressed in Arabidopsis [1].
In the past years, another target of plant metabolic engineering has been to design better and nutritionally enriched crops suitable for large scale human usages. Production of high fructose corn sweeteners from a major yield of U.S. maize crops was carried on. A great impetus was achieved when these high fructose sweeteners were fermented to ethanol. A diverse range of industrially important products like terpenoids, flavonoids, alkaloids, waxes and other FAs can be synthesized out of the metabolic machinery of plants. This requires a genetic modification and transgenic utilization of thevast reservoir of genes in the combined germplasm of the gigantic plant kingdom [2]. Depending on the universality of the genetic code, the introduction of one or more genes like Wax Ester Synthase and Fatty Acyl Reductase genes from Mus musculus into plants is being performed. The aim is to increase the yield of novel valueadded products from plants [3-4]. The criteria for the selection ofthe host plant species for incorporating foreign genes for essential overproduction of value added lipids mainly depends on the evolutionary distance between the host and the donor species. Though there are reports regarding the incorporation of genes into hosts from an evolutionarily distant donor, the success rate is extremely limited. The hosts chosen should have the capability to accommodatethe metabolic load of the incorporated novel gene. Thus, metabolic engineering requires a detailed study of the host metabolic system at the molecular and biochemical levels. Plants are autotrophs capable of naturally synthesizing several complex chemical precursors. Thisindigenous property of plants is portrayed as an advantage over the bioproduction of complex chemicals from heterotrophic microbes. Mass production of complex chemicals in plants is cost effective and is regarded as an eco friendly process [5]. Polyhydroxyalkanoates,being bacterial polyesters, were also synthesized in plants by merging the bacterial metabolic pathways into their plant counterparts [6].
Almost 70% of the world’s edible fat production is fromvegetable oils (triacylglycerols). Plant breeders have used the diverse intraspecific variations in triacylglycerol compositions to breed oil-rich cultivars. Due to concerns about erucic acid (22:1n-9) being associated with myocarditis in rats, dietary erucic acid was eliminated from rapeseeds. Serum cholesterol and coronary heart diseases are tackled well by the intake of saturated FAs (12:0-16:0). This positive correlation first aroused a new vigour among scientists to overproduce saturated FAs in plants. The U.S. Surgeon General’sOffice recommends a healthy diet to consist of about 10% of saturated FAs, 10% monounsaturated FAs (MUFAs) and 10% polyunsaturated FAs (PUFAs). This created a stir among several segments of the food industry. They urged to formulate a healthy diet for consumersvia lipid metabolic engineering [7]. Since then, strategies are being chalked out to replace existing fat sources like hydrogenated coconut and palm-kernel oils with less saturated, partially-hydrogenated oilsubstitutes from the field. It is also necessary to define the genetic control over plant lipid compositions and the effect of genetic variations over “structured lipids” [7].
Maintenance of prescribed lipid compositions with necessary percentages of saturation or unsaturation is a major target. Collecting knowledge about ways to synthesize FAs which are generally notfound in cultivars has been a major challenge in the field of metabolic engineering. The γ-linolenic acid (18:3n-6, 9, 12) is normally absent in the fats and oils derived from general cultivars and field crops. This oilobtained from selected plants like the evening primrose, has shown its medicinal value in atopic eczema. Cost effectiveness via creating transgenic varieties by the use of medium chain triglycerides (MCTs) has been a step to introduce such novel FAs in the lipid content of general field crops [8].
Reduced availability of primary stock fish oils has diverted metabolic engineering in designing plants as alternate sources of omega-3 (n-3), long chain PUFAs (LC-PUFAs) [9-10]. The evergrowing population has presented a consumption level, which has far exceeded the capacity of n-3 LC-PUFA production by algal orgenetically modified bacterial fermentation [11]. Though technically challenging, the low cost and the probability of large scale production has popularised researches in synthesizing n-3 LC-PUFAs in terrestrial crops [12]. Higher plants have no capacity to produce these FAs. Recent alterations in their metabolic pathways have shownbetter results in improving contents of EPA (eicosapentaenoic acid; 20:5n-3 or 20:5Δ5, 8, 11, 14, 17) and DHA (docosahexaenoic acid; 22:6n-3 or 22:6Δ4, 7, 10, 13, 16, 19) [13]. Such advances have been possible due to the presence of a “substrate-dichotomy” bottleneck,existing between the phospholipid-dependent desaturases and the acyl-CoA-dependent elongases [13-15].
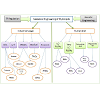
Another important aspect of phytolipid metabolic engineering is the industrial production of waxes and biofuels. The epidermal cells in the primary plant surface are rich reservoirs of waxes [16]. Cuticular waxes are important industrial sources of high-value lubricants, cosmetics, pharmaceuticals and even high-energyefficient biofuel [17]. A small variety of plants generally cultivated in tropical areas have natural wax contents. Hence, the availability and structural diversity of waxes are limited. These shortcomings arebeing tackled by introducing genetically engineered and uniformly cultivable high yielding oil crops, where diversity of wax structure can be attained [17,18]. Biodiesel can be produced from plant oils by transesterification reaction with an alcohol (methanol orethanol) in the presence of a base, an acid or an enzyme catalyst. The production can either be indirect (where the oil is extracted from any oleaginous source followed by in-vitro catalytic trans-esterification) or direct (performed from redesigned cell factories, i.e., through the development of metabolically-engineered plants). Lipid engineering in plants is thus a multifaceted topic. It finds usage both in the industrial sector as well as in modulating human diet. The main components of lipid catabolism which are important to mankind are terpenes, triacylglycerol, wax, octenes, docosahexaenoic acid,margarine, mint, etc (Figure 1). Thus, concentrating on the diverse potentiality of phytolipid metabolic engineering, we would discuss about the detailed advances in this field in the following sections of this review.
Structural composition of FAs in plants
Over one lakh simple and complex compounds add to thechemical diversity of higher plants. Among these, FAs varying in chain lengths, saturation and unsaturation status along with further modifications are found. Generally, the cultivated herbaceous terrestrial green crops consist of FAs which are 16 or 18-carbonlong. The saturation is found in the 16C FAs and around none to about three cis unsaturations are found in the 18C FAs. Here, double bonds are generally present at the n-9, n-6 and n-3 positions, with exceptions in the oils being derived from evening primrose and borage. These plants contain γ-linolenic acid with unsaturations at n-6, n-9 and n-12 positions, as has already been mentioned earlier.The other major classes of oil like palm-kernel and coconut oils have saturated FAs, whose chains constitute less than or equal to 14C [19]. Mutagenesis can also be used to promote diversification of oils in plants. Linoleic acid (18:2n-6, 9) was converted to oleic acid (18:1n- 9) by a single mutation in sunflower. The combination of induced mutations produced a line with low linolenic acid and high linoleic acid, with a decreased concentration of unsaturated FA and an increased concentration of the less saturated precursor.
Mutations also negatively affect lipid composition in plants. Since the oil bodies in plants only participate in storage functions, the plant can tolerate a wide diversification in the fat content. Ample evidences however proved this to be wrong. The mutations as discussed, could either create deficiency in specific FA desaturases (FADs) or block the activity of some component in the triacylglycerol synthetic pathway that makes the availability of FA for desaturation [20]. The same desaturases acting upon both membranes and storagelipids pose a great problem. Problem has been observed in the case of Arabidopsis mutants wherein the unrequired alterations in the structural composition of storage lipids have led to the deleterious effects in membrane lipids [2,20].
Lipid biosynthesis
FA biosynthesis
Malonyl-CoA produced by acetyl-CoA carboxylase (ACCase)acts as the central carbon donor for FA biosynthesis. The malonylgroup is transferred from CoA to a protein cofactor, acyl carrierprotein (ACP) before entering the FA synthesis pathway. The growingacyl chain is joined via a thioester bond to the phosphopantheinprosthetic group of the ACP. The ACP is a 9 kDa small acidic protein.Condensation reactions occur between the malonyl-thioester andacyl-ACP acceptors with the release of CO2 which drives the reactionforward making it irreversible [21].
Three separate condensing enzymes called 3-ketoacyl-ACPsynthases (KAS) help in the production of an 18C FA. A 4C product is formed by the first condensation between acetyl-CoA and malonyl- ACP, catalyzed by KAS III. KAS I is the second condensing enzyme which aids in the formation of FAs, having 6 to 16 carbons. Elongationof the 16C palmitoyl-ACP to stearoyl-ACP requires KAS II [21]. The 3-ketoacyl-ACP is the initial product of each condensation reaction. In order to form saturated FAs, three more reactions take place after each condensation reaction. The 3-ketoacyl-ACP is enacted upon by3-ketoacyl-ACP reductase to get reduced at the carbonyl group. The enzyme uses NADPH (reduced Nicotinamide Adenine Dinucleotide Phosphate) as the electron donor. Dehydration reaction then occurs catalyzed by hydroxyacyl-ACP dehydratase. Enoyl-ACP reductase then completes each cycle of FA synthesis, using either NADH(reduced Nicotinamide Adenine Dinucleotide) or NADPH to reduce the trans-2 double bond. These reactions lengthen the chain of the FA by two carbons, while the FA still remains attached to ACP as a thioester [21].
About 75% of the FAs in plants are unsaturated, though the biosynthetic pathway produces saturated FAs. The soluble enzyme stearoyl-ACP desaturase aids in the formation of the first double bond. The speciality of this desaturase is that it is absolutely unique to the plant kingdom. The other desaturases reported are integral membrane proteins, whereas stearoyl-ACP desaturase is soluble in nature. X-ray crystallography showed that this enzyme is ahomodimer with each monomer consisting of an independent active site with a di-iron-oxo cluster. A central four helix bundle forms co-ordinate bonds with two iron atoms. The motif D/E-E-X-R-H is found in two of the four helices. Hydrogen is abstracted from the C-H bond by a high valent iron-oxygen complex, formed by the binding of the reduced iron centre to oxygen [21].
Removal of the acyl group from ACP results in the termination of the elongation of FAs in the plastids. Generally, an acyl-ACP thioesterase releases free FA by hydrolyzing the acyl-ACP. Alternatively, transfer of the FA from ACP to glycerol-3-phosphate or to monoacylglycerol-3-phosphate is carried upon by one of thetwo plastidic acyltransferases. Oleoyl-ACP is the preferred substrate of the first acyltransferase, which is soluble in nature. The inner chloroplast envelope membrane contains the second acyltransferase,which prefers palmitoyl-ACP as its substrate. The next destination of the FA ensures whether the FA will be released from ACP by a thioesterase or by acyltransferase. Action of thioesterase frees the FA, which then leaves the plastid. Transport of FAs through the lipid membrane of the plastid is made possible presumably by diffusion. An acyl-CoA synthetase assembles an acyl-CoA thioester on the outer membrane of the chloroplast envelope. This assembly participates in acyltransferase reactions to synthesize glycerolipids in the endoplasmic reticulum [21].
Recently, a group of researchers have isolated a family of chalcone isomerase (CHI)-like genes. These genes encode for FA-binding proteins, which aid in the transport of FA to the outer envelopeof the plastid. Thus, this proves to be a new target for metabolic engineering of lipids in plants. Higher lipid production is expected by overexpressing CHI-like FA binding proteins in plant cells. The CHI-likeFA binding proteins and genes can be modified in recombinant cells of transgenic plants to improve lipid production at par with industrial demands [22].
The Arabidopsis mutants defective in the plastid enzyme, glycerol-3-phosphate acyltransferase showed higher accumulations of 18C FAs. This experimental data enhances the fact that the 16- to 18-carbon FA ratio is maintained by the regulated activities ofthe elongase, acyltransferase and hydrolase [8]. Apart from the general 16- and 18-carbon FAs, some genus like Cuphea accumulate triacylglycerols containing FAs of chain lengths less than 16:0. These medium chain triacylglycerols (MCTs) are abstracted from coconutoil, Cinnamomum camphora drupes, palm-kernel oil and have high physiological value. This is due to the fact that MCTs passively diffuse from the gastrointestinal tract to the portal system, without the subsequent modifications as required by the longer FA chains. Elucidations of the pathways which aid in the formation of MCTs inCuphea are in progress. Accomplishment of a complete database of the metabolic pathway of MCTs in Cuphea will help in targeting and cloning genes to prepare transgenic plants for the overproduction of MCTs [23].
Triacylglycerol (TAG) biosynthesis
Steryl esters (SE) and TAG are produced by the respective esterification of FA-CoA, produced by de novo synthesis with glycerol and sterol. The TAG formation is facilitated by the acylation of glycerol-3-phosphate (G3P) by multifaceted acyltransferases. The G3P may be produced from glycerol by glycerol kinase or by dihydroxyacetone phosphate (DHAP) in a reversible reaction [24]. The TAGs can be stored as neutral lipids or cytosolic lipid droplets. Immediately after seed germination, these TAGs are dedicated to fuelthe growth and seedling development, via the Kennedy pathway. The TAGs synthesised from acyl-CoAs and glycerol is loaded into the endoplasmic reticulum [25].
Introduction of better hosts for crop metabolic engineering
Metabolic engineering has provided the opportunity to design the accumulation and production of novel FAs in plants. These novel FAs should possess important functional groups, beneficial to mankind in general. Arabidopsis has remained a model plant to testthe effects of individual genes and their combinatorial implications in the metabolome for enhancing seed oil content. More recently, oil production in vegetative tissues is a source of great interest [8]. Another emerging attractive host of metabolic engineering is Camelina sativa, as it can be easily transformed using an Agrobacterium-based floral infiltration method [26]. For LC-PUFA engineering, stacking of numerous pathway genes is essential. This is found to be easier in Camelina due to its short life cycle [27]. Nicotiana benthamiana isadopted for the transient leaf assay, widely used in plant sciences. One of the applications is the swift interaction and network of the complex multigene pathways that produce FA profiles. Accurate silencing ofendogenes and overexpression of transgenes in Nicotiana has the potentiality to improve such assays [28].
Lipid metabolic engineering of industrial products
Enriching seeds with oils of industrial importance
Hydroxy and epoxy FAs are used as lubricants, nylon precursors and plasticizers. Extensive researches have been carried out to generate these FAs from non-agronomic plant sources to terrestrial oilseed crops [8]. Functionally divergent n-12 desaturases (FAD2) is an important enzyme in the metabolic pathway for the synthesis of unusual FAs. Production of ricinoleic acid (12 OH-18:1n-9) and the related C18-C22 ω-6 hydroxylated FAs are the most studied. The FAD2 hydroxylases use oleic acid bound to phophatidylcholine as substrates [29]. The castor bean (Ricinus communis) accumulates 90%of ricinoleic acid through this pathway. About 20-30% of ricinoleic acid was found to be accumulated in transgenic Arabidopsis seeds by the transfer of castor FAD2-related hydroxylase and castor acyltransferases, including the castor diacylglycerol acyltransferase 2 (DGAT2) and phospholipid-diacylglycerol acyltransferase1 (PDAT1)[1,30]. Significant increase in most of the major TAG species found in native castor bean oil was observed by making comparisons between single- and double- transgenic lines. This also pointed to the fact that RcDGAT2 greatly modified the TAG pool. The RcDGAT2 showed inclined preference towards acyl-CoA and diacylglycerol (DAG)substrates, containing hydroxyl FAs [1]. The JcDGAT1 and JcDGAT2 from Jatropha curcas, overexpressed in yeast mutant H1246 strain restored the TAG biosynthesis. Overexpression of the same genes also enhanced the biosynthetic quality by 16.6% and 14.3% respectivelyin the INVSc1 strain. The JcDGAT1 and JcDGAT2 overexpression in transgenic tobacco increased the seed oil content by 32.8% and 31.8% respectively [31]. The Arabidopsis Reduced Oleate Desaturation mutants (rod1) were deficient in phosphatidylcholine diacylglycerolcholine phosphotransferase (PDCT)-mediated flux of DAG through phosphatidylcholine (PC). Thus, these mutants showed much reduced FA accumulation in the seeds which were also modified for the expression of castor bean hydroxylase [32]. Replacement of AtPDCT gene with castor bean PDCT increased hydroxyl FA accumulation [8].
Manipulation of an increase in the plastidial ACCase activity was a complex phenomenon due to the multigene-encoded enzyme complex and subsequent post-translational regulations. About 5% increases in oil content was noted when cytosolic version of theenzyme was targeted to the rapeseed chloroplast [33]. Expression of KAS III from Spinacia oleracea in Brassica napus increased the palmitic acid proportion though a decrease in total FA content by 5-10%. A swapping 40% increase in rapeseed oil content was seenby the introduction of Saccharomyces cerevisiae G3p dehydrogenase (gpd1). Arabidopsis recorded 10-21% increase in seed oil content when Carthamus tinctorius acetyltransferase (GPAT) was overexpressed.The Arabidopsis DGAT1 (Diglyceride Acyltransferase 1) increased the oil content and seed weight when expressed in rapeseeds [33].
Several works on Escherichia coli cyclopropane synthase brought to knowledge the conversion of the n-9 double bond of oleic acid into a cyclopropane ring catalyzed by the enzyme incorporated in transgenic lines. This has conferred a wide range of industrial functionson vegetable oils [34]. Co-expression of E. coli cyclopropane synthase with a lysophosphatidic acid acyltransferase (LPAT) from Sterculia foetida seeds was the cause of high cyclopropane FA accumulation.In the TAG biosynthetic pathway, the LPAT catalyzes the acylation of the sn-2 position of the glycerol backbone [34]. Efforts to produce novel oils of industrial importance have also been made by targeting pathways using acyl-CoA as substrate. Crambe seed oil is a C22 monounsaturated, very long FA, which has high erucic acid content (22:1; 60% of total oil content). For production of this high erucic acid vegetable oil, a modified protocol was adopted. It contained the promoters: FAD2 RNAi transgene for increment in oleic acid content; Brassica napus Fatty Acid Elongase1 (FAE1) to improveoleic acid elongation to erucic acid; and Limnanthes douglasii LPAT (lysophosphatidic acid acyltransferase) to enhance erucic acid incorporation in sn-2 position of TAG [8,35]. This resulted in about 73% increase of erucic oil in the top performing lines [36]. A low PDCT activity in Crambe seeds precludes the exchange of FA between DAGand PC. This character of Crambe seeds allows the accumulation of erucic acid through acyl-CoA reactions [37]. Erucic acid is usually synthesized at the later developmental phases according to latest labelling studies. Modern researches aim at the production of this FA at the initial stages of development [8,37]. Erucic acid is unsuitable for human consumption. Hence, low erucic acid rape (LEAR) was developed for food consumption. The cross contamination betweenLEAR and HEAR (high erucic acid rape) has been minimised via “identity preservation” [38]. High erucic acid Brassicaceae lines with increased content of erucic acid and very long chain FAs (VLCFAs) were created by the manipulation of the expression of the endogenousFAD2 gene in Brassica carinata, using co-suppression and antisense approaches [39].
It is important to modify the degree of saturation of FAs, as a particular industrial FA should have uniformity in desaturation and chain length. However, for edible FAs, consistency along with uniformity is required to be maintained, as a mixture of several complex compounds constitutes the desired product. Antisense repression of the Δ9-desaturase gene in rape resulted in higheraccumulation (from 1-2% up to 40% of total FAs) of stearic acid (18:0) at the expense of oleic acid. Thus, the content of oleic acid markedly decreased. The stearic acid can be used as a substitute of cocoa butter. About 80% accumulation of oleic acid has been obtained through conventional breeding programs, but the urge toproduce higher amounts has produced mutants with low tolerance towards cold. This was due to the dearth of unsaturated FAs in the plasma membrane. However, 87-88% oleic acid accumulation was recorded using antisense repression and co-suppression of the rapeseed Δ12-desaturase gene. The cold tolerance of these plants was not weakened [8,40].
Several interesting modifications of lipid metabolism in transgenic plants have been performed in the last decade. These modifications led to the production of important oil products which possess a large share in the market even today. Medium chain lauric acid (12:0) is overproduced in coconut and palm-kernel to be used insoaps, detergents and surfactants. Conversion of 24% of total FA to laurate was facilitated when the Umbellularia californica lauryl-ACP thioesterase was expressed in Arabidopsis. The same thioesterase expressed in rapeseeds showed conversion of 58% of total FA to laureate. Cuphea hookeriana FatB1 thioesterase co-expressed withLPAT of Cocos nucifera in Brassica napus converted 67% of total FA to laurate [33]. Long chain erucic acid about which we have already discussed is used widely in industrial lubricants. The hydroxylipid, ricinoleic acid extracted from castor bean is used in coatings andlubricants. Trienoic lipid, linolenic acid overproduced in flax has been used in paints and varnishes. Vernolic acid has remained a low cost oil of high industrial importance. This is due to the possible conversionof this FA into polymers like PVC (polyvinyl chloride), paints, terpenes and lubricants. This epoxy lipid extracted from transgenic epoxidized soybean oil, is used as plasticizers. The (+)-Vernolic acid (cis-12, 13-epoxy-octadec-cis-9-enoic acid) is the first naturalepoxy FA to be isolated from the oil of Vernonia anthelmintica seeds. The (-) stereoisomer of vernolic acid has been isolated from some seed oils of Malvaceae. The biosynthesis of this FA is mediated by an epoxygenase enzyme, targeting which can lead to over 60%accumulation of (+)-vernolic acid in the seed oil of V. galamensis [41]. Crepis paleastina Δ12 epoxygenase (Cpal2) expression in the Arabidopsis fad3/fad7-1/fad8 triple mutant resulted in a 1.6 fold increased vernolic acid accumulation in the Arabidopsis seed oil [42].The Arabidopsis fad3/fae1 double mutant was found to be defective in the microsomal Δ15 desaturation and oleic acid elongation, resulting in higher levels of ALA (α-linolenic acid). Co-expression of Cpal2 epoxygenase, along with C. paleastina FAD2 Δ12-desaturases genesin Arabidopsis fad3/fae1 mutant showed vernolic acid, consisting of 21% of the seed oil. Transgenic cotton seed oils constituted 16.9% of vernolic acid as a result of the co-expression of Cpal2 epoxygenase and CpFAD2 genes in cotton. The expression of Cpal2 epoxygenaseshowed a small increase in oleic acid accumulation. This relieved the cotton plant from the build up of oleate due to its appreciated endogenous activities to edit the unusual FAs from phospholipids [42].
Metabolic engineering of ω-7 FAs in plants
Palmitoleic acid (16:1Δ9) and cis-vaccenic acid (18:1Δ11) are ω-7 FAs which can be metabolically engineered in plant oils. These can be utilised as feedstocks in metathesis chemistry to produce theindustrially important octene [43]. Higher accumulations of such ω-7 FAs were achieved by metabolic engineering of plastidial 16:0-ACP desaturase into Arabidopsis thaliana. This enzyme increased the formation of palmitoleic acid with increase in specificity of the efficientconversion of 16:0 to 16:1Δ9 by about 100 folds in comparison to naturally occurring paralogs in Doxantha ungis-cati. Accumulation of ω-7 FAs increased from < 2% to 14% by the expression of the engineered enzyme, Com25 in the seeds. Combinatorial saturation mutagenesis or selection to identify variants with better functioningtowards acyl chains of < 18C in length aided in the isolation of Com25 (a variant of Ricinus communis) [44]. Down regulation of KAS II 16:0 elongase lowered the competition for 16:0-ACP which rapidly increased the ω-7 FA content to 56%, with 21% of the 16:0 FAs leavingthe plastids without desaturation. This exodus was reduced to 11% by the co-expression of two fungal 16:0 desaturases which increased the ω-7 FA accumulation levels to 71% [43].
Metabolic engineering of Sandalwood oil
Santalum album (of family Santalaceae) heartwood oil has remained a high demanding raw material of perfume industries. However, this valuable group of plants have got their names registered into the vulnerable category of the IUCN (International Union for Conservation of Nature and Natural Resources) Red List [45]. The main problem of sandalwood trees is that these are slow growing woody perennials and metabolic engineering is the only resource to design heterologous production systems. Santolols, bergamotols and other sesquiterpene compounds are the characteristic ingredients of sandalwood oil. The farnesyl diphosphate synthase (FPPS) catalyse the synthesis of farnesyl diphosphate (FPP) from dimethylallyldiphosphate and isoprenyl diphosphate. This is the first step recorded in the biosynthesis of santalol and bergamotol [46]. The santalene synthase (SaSSy) cyclises FPP into a mixture of santalenes and α-exobergamotene;these santalenes and bergamotenes have been foundto be oxidised to santalols and bergamotols by a multi-substrate cytochrome P450 dependent monooxygenase (P450). Recently a new CYP76F subfamily in Santalum album was identified through transcriptomic screenings of recombinant P450s. This subfamily has been found to be involved in the increased biosynthesis of α, β,and epi β-santalols and bergamotols [46]. The CYP71 clan consists of the CYP76 gene family and the P450 families which have ample connections with primary and secondary metabolism. Ten S. album P450s and an NADPH-dependent cytochrome P450 reductase (CPR) have been found to increase the efficiency of santalol and bergamotolbiosynthesis. Cloned santalene synthases, along with CYP76 and CPR cDNAs have been posed as potent tools to highly increase sandalwood oil content. Metabolic engineering ushered the production of santalols and bergamotols by the expression of SaSSy and SaCPR (combined with multi substrate SaCYP76Fs) in yeasts [46,47]. Genetic markerfor detailed overview of the tree improvement and oil synthesis has been proposed out of cloned terpene synthases and P450s [48,49]. An exhaustive approach to study the biosynthetic pathway of santalols indetail was recently taken up wherein it was observed that development of the plant could regulate the isoprenoid (sesquiterpenoid being a C15 isoprenoid) pathway in a tissue specific manner. The gradual accumulation of isoprenoid pigments in the plastid along withreduced levels of farnesyl intermediates was observed. However, with maturation, it has been reported that the isoprenoids got converted to sesquiterpenoids [50]. Hydroxy-3-methylglutaryl CoA reductase and 1-deoxyxylulose-5-phosphate synthase exhibited differential expression patterns while possibly mediating post-transcriptional regulation. The farnesyl pyrophosphate, sesquiterpene and monoterpene synthase transcript levels were higher in the callusthan in the matured leaves, providing a hint in the tissue specificity [50]. This was the first approach to make a detailed blueprint of the biosynthetic and metabolic pathways of isoprenoid in S. album at the molecular level. Further advancements in this field will open the scope to target specific enzymes in the pathway to get a higher yield of this industrially valuable oil.
Wax production for industrial usage
Importance of waxes
Cuticular waxes found in plant surface epidermal cells belong to the family of VLCFAs and are C20-C34 straight chain aliphatics. They also consist of alicyclic and aromatic compounds like triterpenoids, alkaloids, phenylpropanoids and flavonoids in some plant species.Presence of waxes in plants plays a pivotal role in the protection against water loss, UV rays, pathogens, insects and other biotic and abiotic stresses [17]. Waxes are lipophilic compounds which remain solid at room temperature. These can be transparent to opaque and can be easily polished. Due to desirable physical properties such as hardness, cure speed, melting point, pour point, viscosity, low surface tension, adhesive strength, optical transparency, durabilityand thermal expansion co-efficient, waxes are used as lubricants, adhesives, coatings, sealants, impregnation materials and adjuvants in the designing of biologically active compounds and experimental setups [51,17]. Final finishes on automobiles, textiles, pesticides,candles, cosmetics, dental pharmaceutical products, drugs (lozenge coating), chewing gums and confectionary products all rely on waxes.
Possibility of wax production in seeds
Realizing the immense importance of wax in different industrial sectors for varied applications, there is a great demand to overproduce it from plants. In this context, genetic engineering has opened up newavenues for wax overproduction on plant surfaces. Introduction of wax biosynthetic pathways into oilseeds has led to the production of waxes, constituting optimal structural stabilities to be used in industrial products. One of the major plant oil commodities todayare waxes [17]. Reports suggest that the wax yields from Brassica seed oil range between 500 to 4000 Kg ha-1 and those from Canadian canola ranges from 1500 to 1800 Kg ha-1 [52]. Generally, the wax accumulation is much less, compared to the accumulated oil in seeds, but the baseline is that there is several folds increase, compared to the natural cuticular waxes. Jojoba (Simmondsia chinensis) plant is the only known species which naturally accumulates wax in its seeds (75-750 Kg wax ha-1) [53].
Overproduction of waxes by metabolic engineering
The rational and cost-effective approach towards waxoverproduction in transgenic plants is the identification and proper database knowledge of the target wax biosynthetic pathways. The effect of each step of each pathway needs to be vividly studied in transgenicplants [17]. Following gene cloning, the effects are studied in a plant species for the enzyme encoded by it [54-56]. The initial reactions catalyzed by FAE in the wax biosynthetic pathway are common to those which include the synthesis of sphingolipids [57]. Sphingolipidsare the necessary components of cell membrane in all plants. Thus, VLCFA precursors for wax production are usually found in all tissue cells of host plants. Transgenic variation of the levels of fatty acylreductase (FAR) and wax ester synthase (WS) leads to wax ester accumulation. The down regulation of competition often enhances the target product formation. The TAG biosynthesis competes for the FA precursors. Hindering this pathway essentially increases wax levels in the cells. Increased synthesis of wax and steryl esters has been reported in transgenic plants wherein the steroid biosynthetic pathway was up regulated. Modifications of wax by further co-expression of enzymes like hydroxylases, desaturases, etc., bring diversification in wax products [17]. Similarly, there are evidences wherein waxesters produced in jojoba have been metabolically engineered in the oilseeds of Crambe abyssinica [54,58]. According to experiments performed with Arabidopsis stem wax, the ester biosynthesis in the epidermal cells was found to be checked by wax alcohol pools, thoughthe particulars of enzyme specificity remained unknown [59]. The knowledge about the specific substrates of the enzymes will accelerate the production of diverse waxes by designing improved transgenic crops. Metabolic engineering also allowed the inclusion of artificialdeliberate enzymatic steps which naturally never occur in order to maximize wax production [17].
Waxes with broad industrial applications consist of esters containing acyl and/or alkyl moieties with C = C bonds, cyclopropane rings and methyl branches. Such structural features have been observed to be biosynthetically related in the spikes of barley [60].Specificity of the position where the unsaturation (double bond) is to be created is determined by a desaturase. The branching in the structural configuration is induced by a cyclopropane synthase and/or a methyl transferase. Another important component of wax is the ricinoleic acid, mainly found in the castor bean, as has exhaustivelybeen discussed in one of the previous sections. Metabolic engineering is essential in the castor beans for the production of waxes. This is due to the fact that castor beans are not at all ideal sources of rearingwax due to requirement of costly manual harvesting and presence of complex allergens, along with the presence of a toxin, ricin [61]. The enzymology of wax biosynthesis is not as complex as TAG biosynthesis. Hence, the designing of potent transgenic crops capable of incorporating ricinoleic acid into wax esters are being carriedout [17]. Recent findings indicate that the derived cuticular waxes are synthesized in the endoplasmic reticulum (ER) of the epidermal cells before they are exported to the outer face of the epidermis. Thetransporters required for such wax export has been known, but the knowledge about the intracellular and extracellular wax trafficking is yet to be ventured [62].
Production of waxes through genetic engineeringapproaches overcoming the kingdom barrier
Generation of unusual wax esters has been made possible in transgenic plants by harbouring genes from a wide variety of organisms including mammals, birds, fungi and bacteria [63-67]. Long chain length monounsaturated wax esters were produced in Arabidopsis by introducing FAR and WS genes from mouse (Mus musculus) [18]. The MmFAR1 and MmWS enzymes, in spite of having the desired substrate specificities, fail to co-operate in vivo, notexpressing similar subcellular localizations [68-69]. The peroxisomal targeting signal (PTS) is absent in MmFAR1. Still, the enzyme localizes in the peroxisome. This has been accorded possibly to the presence of a stretch of 66 amino acids at the C-terminal, acting as a membrane anchor. The uniqueness of MmFAR1 lies in the fact that itis possibly the only enzyme which is targeted to the peroxisome by a C-terminal transmembrane anchor, which obviously is not a part of the peroxisomal protein translocation complex [18]. The astonishingfact is that the peroxisomal targeting of MmFAR1 is highly conserved in varied organisms from mouse to onion. The different localizations of MmFAR1 and MmWS in mouse and onion epidermal cells fail toportray a defined pathway for production of wax esters. Thus, the two genes were co-targeted by fusing them with Arabidopsis oleosin 3 (Oleo3). This was done to prevent alternate reactions hindering wax production by proper channelling of lipid intermediates by thejoint association of the two enzymes in one membrane or even in one membrane microdomain [18]. Such a protocol was also maintained for accumulation of α-eleostearic acid in the oil of tung tree seeds. The high degree of unsaturation in α-eleostearic acid is responsiblefor its utility as a drying oil, immensely important in the preparation of paints and varnishes [70].
Greater accumulation of wax esters was found by co-targeting Oleo3: MmFAR1ΔC and Oleo3: MmWS, rather than the unmodified enzymes together. It was said that such truncation in the C-terminus did not decrease the catalytic efficiency of the co-targeted enzymecomplex but increased the spatial proximity. Comparison of wax esters produced on co-expression of Oleo3: MmFAR1ΔC and Oleo3: MmWS in yeast and Arabidopsis with those of mCherry: FAR1 (mCherry is a fluorophore) and EYFP: MmWS (EYFP-Enhanced Yellow Fluorescent Protein) was analysed. The analysis showed greater accumulation of wax esters in yeast compared to Arabidopsis. This was probably because yeast naturally contains a high amount of oleate, which acts as an easy substrate for MmFAR1 and MmWS[18]. Elimination of FA-modifying enzymes like FAD and FAE in Arabidopsis fae1 fad2 double mutant produced greater than 65% oleyl-oleate. Oleyl-oleate is a molecular wax ester species, which has its content around 5% in the wild type Arabidopsis [18].
Issues hindering wax overproduction in transgenic crops
The transgenic lines have low germination rates and intracellular autotoxicity of waxes develops in seed embryo cells, often prohibiting seed germination. In natural conditions, the accumulation of waxes innormal quantities in the epidermal cells helps the waxes to be exported to the plant surface [71-72]. The relatively short chain wax esters with unsaturated acyl and alcohol moieties are found in the jojoba oil.Thus, these wax esters have a low melting point, leaving them to be liquid at room temperature, whereas the fully saturated longer chain esters are solid at room temperature. Thus, the prominent presenceof short chain wax ester-synthesizing enzymes in the biosynthetic pathway could affect the accumulation of longer chain wax esters in transgenic seeds [17].
Phytolipid metabolic engineering as a tool for biofuel production
Reason for requirement of alternative hydrocarbon biofuel source
Chemical synthesis and extraction from fossil sources provide a wide range of alkanes which consist of another section of the verylong-chain wax compounds. Biotechnological methods to produce waxes of this class are not necessary as the production of thesewaxes in the aforesaid process generates wide diversity with costeffectiveness [12]. Other than uses as traditional industrial waxes, these long chain hydrocarbons have illustrated a bright future beforeus as potent biofuels. The transport sector mainly uses gasoline and petroleum as fuels, as the highly reduced carbon content within them contains the maximum chemical energy [73]. Ethanol and biodiesel have shown the ability to replace petroleum in some aspects. Due to the extent of energy retained, these hydrocarbons still play pivotalroles as fuels [12,74]. The alternative hydrocarbons are required, as the traditional sources are gradually depleting [17]. Biologically derived fuels are eco-friendly, causing lesser environmental hazardsand are economically feasible.
Production of hydrocarbon-rich biofuel
The main biotechnological target of synthesizing biofuel is the epidermis of higher plants. Here, the plant can transform the FAs into the hydrocarbons which gradually get accumulated in the cuticularwax, deposited on the plant surface. The level of natural hydrocarbon formation in the cuticular wax is insignificant for industrial use. New enzymes are required which can enhance the biosynthesis of thesehydrocarbons at the proposed site. Study of hydrocarbon formation and the isolation of essential genes for bio-gasoline production can be done in this proposed model system [17]. It is thought that the loss of one carbon atom occurs from the acyl precursors during the transition step of the central reaction of the alkane formingpathway. This transitional step includes the conversion of odd numbered carbon chains from even numbered. Cloning of genes like CER1, CER2, CER3/WAX2 (controlling the formation of cuticular lipids) has been performed, though the essential increase in the hydrocarbon content needs further researches. The conversion of FAs to hydrocarbons requires multiple transducing steps. So, a completedatabase consisting of the blueprint of this signaling pathway, along with the participating enzymes is needed for the scope of metabolic engineering in this aspect [17].
Metabolic engineering poses TAG as a potent biodiesel
TAG engineering in higher plants
Genetic modifications and designing of transgenic crops have opened the possibility of oil production in vegetative tissues in orderto squeeze out the maximum energy from plant biomass. Reports have shown the capability of certain tissues to produce TAGs and lipid droplets which have been spotted in the cytoplasm of mesophyll cells in leaves [75]. High accumulation of TAGs is found in senescentleaves experiencing stress. Still, the vegetative tissues have a low content of TAGs [76-77]. Metabolic engineering can increase TAG accumulation. This has been attained by the ectopic expression of biosynthetic enzymes like acyl-CoA: diacylglycerol acyltransferase (DGAT) or monoacylglycerol acyltransferase (MGAT); genes suchas LEAFY COTYLEDON1 (LEC1), LEC2 or WRINKLED1 (WR1)controlling seed development and maturation [78-80]. The TAG content can also be increased by mutating genes involved in FA turnover, like COMATOSE2 (CTS2), SUGAR DEPENDENT1 (SPD1) or COMPARATIVE GENE IDENTIFICATION-58 (CGI58) [81-82,8]. Increased levels of storage lipids in leaf biomass can be achieved by re-orienting the carbon flux into TAG, while overexpressing LEC2 in the cts2 β-oxidation mutant [83]. Non-seed proteins which possessthe inherent ability to bind and stabilize lipid-rich particles in the cytosol are becoming new targets of metabolic engineering [83].
TAG engineering in algae
Metabolic engineering has ensured increase in the yields of biofuel-related lipids in microalgae without compromising growth [84]. Targeted knockdown of a multi-functional lipase/phospholipase/acyltransferase was performed. This showed an increment in the lipid yield of the diatom Thalassiosira pseudonana, without affecting its growth rate and pattern [84]. Antisense-expressing knockdown was done on the strains 1A6 and 1B1 of the diatom. The modified strains exhibited a growth pattern similar to the wild type and an increased lipid content of 2.4- and 3.3- folds under simultaneous light to alternating light/dark conditions and after 40 hours of silicon starvation respectively [84]. The knockdown strains showed similarities with the wild types in TAG depletion during growth. However, the knockdown strains contained more TAG throughout the exponential and stationary phases. Transcriptomic analyses have shown variable expression levels of lipases. Thus, diverse and independent activities of specific TAG lipases are thought to usherthe analyses in guiding targeted manipulations [84]. Preliminary transcriptome analysis and overexpression of endogenous genes also showed increased lipid accumulation in the diatom, Phaeodactylum tricornutum [85].
Transesterification of TAGs results in biodiesel which is monoalkyl (methyl) esters (FA methyl ester, or FAME). Such green fuel has higher flashpoint which allows safe handling, storage and optimum lubricacy required in engines. In order to modify the red algae,diatoms and dinoflagellates, several general transformation protocols like particle bombardment, silicon carbide whisker agitation and Tiplasmidinsertion have been adopted. The efficiency of expressiondepends on auto-attenuation of the exogenous sequences, codon usage bias, GC content and the proteasome-mediated degradation [86]. Some green algae have a tendency to accumulate high levels of TAGs, while facing nitrogen starvation [87]. The baseline strategiesadopted for metabolic engineering in algae are to overexpress TAG biosynthetic enzymes; making an increment in the availability of precursor molecules like acetyl-CoA; inhibition of lipase-mediatedhydrolysis and β-oxidation to arrest FA catabolism; changing the saturation profiles via regulating desaturases and finally the optimization of TAG FA chain length, via effective control of thethioesterases. Identification of 80 mutants with altered FA synthesis activities in a large scale mutant screening of Chlamydomonas reinhardtii insertional library enhanced the complexity of lipid metabolism in algae [23]. C. reinhardtii and Volvox carteri are important laboratory models used in developing algal biofuel, since efficient expression of transgenes and gene regulation, via useof riboswitches can be performed. Inducible nuclear promoters, luciferase reporter genes and inducible chloroplast gene expression can also be favourably carried out in these models [88-89,23]. The TAG biosynthetic enzyme-coding genes can be knocked down in algae using the high-throughput artificial miRNA (amiRNA) technique, which is highly stable and specific [90]. TAG accumulation has also been reported in the freshwater algae Scenedesmus sp. LX1. A relation between low temperature and higher accumulation of reactive oxygen species in the microalgal cells was also chalked out in this research [91].
TAG engineering in fungi
Microorganisms with the capability to accumulate lipids at a level above 20% of their biomass are termed oleaginous in nature. Some species of yeasts and filamentous fungi can accumulate TAG ranging even up to 70% of their biomass. Significant attentionshave been drawn by the use of such heterotrophic oleaginous microorganisms to produce single cell oil (SCO) [92]. Fungal SCOs have a short process cycle and production is not affected by seasonaland cyclical variations in the abiotic conditions [94]. Oleaginous fungi like Humicola lanuginosa, Aspergillus oryzae, Cunninghamella echinulata, Mortierella isabellina, Mucor mucedo, etc., play pivotal roles in SCO production. Yeasts belonging to the genera Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus and Lipomyces also produce SCOs [93]. They have an inherent property to degrade hydrophobic substrates like n-paraffins and oils and have valuable usage in biotechnological improvements in producing novel biofuels[24]. Nitrogen starvation is the most efficient condition for the induction of lipogenesis. The extra carbon instead of remaining unutilised or converted into storage polysaccharides as in nonoleaginous species, is channelled towards lipogenesis in oleaginous species. This leads to TAG accumulation in the intracellular lipid bodies [94].
The GUT1 encode glycerol kinase which catalyses conversion of G3P from glycerol. The G3P dehydrogenase encoded by GPD1 synthesises G3P from DHAP. The antagonistic reaction producing DHAP from G3P is catalysed by a second isoform of G3P dehydrogenase encoded by GUT2. In Yarrowia lipolytica, GPD1 was overexpressed and GUT2 was deleted to enhance the accumulation of G3P. The six POX genes (POX1 to POX6) encoding the peroxisomal acyl-coenzyme oxidases were deleted to disruptβ-oxidative metabolism to facilitate a metabolic accumulation of lipids [95-96]. Functional expression of heterologous genes has been exploited in metabolic engineering to diversify the range of substratesused by oleaginous fungi [24]. Increased bio-production has been recorded by the use of inulin [97]. Heterologous expression of the Kluyveromyces marxianus exo-inulinase gene (INU1) on a high copy plasmid was performed in Y. lipolytica for lipid accumulation. The inulin was hydrolysed by the expressed inulinase, assimilated andconverted to TAG [24]. To enhance a superior de novo lipogenesis in Y. lipolytica, coupling combinatorial multiplexing of lipogenesis targets, along with phenotypic induction were performed. Tri-level metabolic control resulted in saturated cells which accumulated more than 90% lipid content, showing a 60-fold improvement over parental strain [98].
Biofuel production from wastewater
A potentially important feedstock for production of third generation liquid biofuels like biodiesel (FA alkyl ester) along with high value organic and inorganic resources is portrayed by oleaginous biomass from biological wastewater treatment (BWWT)[99]. Pyrolysis oils (bio-oils) are short chain alcoholic biofuels. The Fischer-Tropsch process has shown the sketch of deriving alternative biofuels from wastewater organisms. Pyrolysis of waste sludge has shown the capacity of producing potent bio-oils. Such oils consist of chains ranging from 6-20 carbons [100]. The genera like Euglena, Oscillatoria, Chlamydomonas, Scenedesmus,Chlorella, Nitzschia, Navicula and Stigeoclonium were found to be the most tolerant ones in wastewater polluted with toxic xenobiotic compounds [101]. Euglena like many other members of Chlorophyceae poses a suitable source of lipids with the potential to act as biofuel [102]. The FA methyl esters (FAME) comprising of C18:3 (31%) > C16:0 (25.2%) > C16:4 (14.8%) > C18:0 (7%) with a total lipidcontent of 27.2% were found to be accumulated in E. gracilis cells, grown heterotrophically. Accumulation of FAME with C18:3 (40%) > C16:0 (16%) > C18:2 (18%) > C16:2 (8.5%) along with 21% lipid was reported in Chlorella pyrenoidesa [103]. C16-C18 (essential) fattyacids have desirable biofuel properties, such as palmitic, stearic, oleic and linolenic acids [104]. The TAGs and wax esters extracted from the waste water bacterial and algal community are the actual sources of such bio-oils, which also contain isoprenoid lipids like farnesene.Metabolic engineering to produce higher levels of such lipids in the waste water microflora are being enacted via engineering of the genes belonging to the TAG and wax biosynthetic pathways [105].
For the sustainable production of biofuel, the concept of a ‘wastewater biorefinery column’ has also been proposed. This can further inspect the utilities concerning the genetic reservoir of waste water microorganisms [105]. Shallow paddle wheel-mixed high rate algal ponds (HRAPs), having higher productivities (about 30 tonnes ha-1) and the capability to promote bioflocculation are being designed [106]. The Offshore Membrane Enclosure for Growing Algae (OMEGA) is a newly evolved strategy by the National Aeronautics and Space Administration (NASA) to produce algal biofuel using photobioreactors (NASA-OMEGA Project). This modern technology still requires exhaustive experimentations posing a grand challenge to microbial ecologists and genetic engineers to extract every percent of biomass from waste water bacteria and algae for efficient conversion to effective biofuels [105].
Metabolic engineering as a tool to produce bioplastics
Structural variations and close analogy to plastics have brought polyhydroxyalkanoates (PHAs) to the centre of attraction in metabolic engineering. These are natural polymers with thermoplastic properties[107]. PHAs are polyesters that have been found to accumulate in a diverse range of bacteria under nitrogen limited conditions with excess carbon. Regulation of quality and improvement over cost-effectivenessare dealt with poly (3-hydroxybutyrate) (PHB) production in plants and other recombinant microorganisms. They are biodegradable and potent elastomeric replacements of conventional plastics [107-108]. The PHA accumulation in transgenic Arabidopsis was successful by the expression of bacterial PHA biosynthetic genes.
The PHB biosynthetic genes phbA, phbB and phbC (encoding 3-ketothiolase, acetoacetyl-CoA reductase and PHB synthase respectively) are clustered together into one phbCAB operon in the bacterial genera [108-109]. A critical parameter with respect to PHB production in plants is the expression of the β-ketothiolase gene, phbA.The chimeric constructs used in tobacco, potato and Arabidopsis held the constitutive expression of phbA for the drastically reduced transformation efficiency [110]. Preventing the expression of phbA was done by putting it under the control of an inducible promoter or separation of the coding sequence. This showed enhanced growth of the transgenic lines. The PHB biosynthetic intermediates(acetoacetyl-CoA and 3-hydroxybutyryl-CoA) or the derivatives of these intermediates, the depletion of the acetyl CoA pool or interactions of β-ketothiolase with proteins, exerted toxic effects on the transgenic plant growth and PHB production. Such problems could be tackled by designing an inducible promoter system or a somatically activated expression system [110]. Important roles inthe size and formation of PHB granules are played by phasins. Three homologues of the phasin protein encoded by phaP1 were identified in Ralstonia eutropha strain H16. The functions of the proteins encoded by phaP2, phaP3 and phaP4 were examined by analysis of R. eutropha H16 deletion strains (DeltaphaP1, DeltaphaP2, DeltaphaP3,DeltaphaP4, DeltaphaP12, DeltaphaP123 and DeltaphaP1234) [111]. Constitutive expression of phaP from R. eutropha, along with the phb genes in Arabidopsis led to higher accumulation of PHB, though the growth was stunted [110]. Another experiment was designed where the phbA, phbB and phbC genes from R. eutropha were placed underthe control of the seed coat peroxidase promoter and were introduced into soybean (Glycine max). Analyses of the seed coats arising from four independent transformation events were performed to assay PHB accumulation. The results showed production of PHB at a mean of 0.12% seed coat dry weight, accompanied with individual valuesup to 0.36% [112].
Enriched insulation properties were achieved by the production of plastic cotton fibres. The enzyme responsible for the first step inPHB synthesis, i.e., 3-ketothiolase is present in the cotton fibres. So the acetoacetyl-CoA reductase and PHB synthase encoding genes (phaB and phaC respectively) of Alcaligenes eutrophus was introduced into cotton by particle bombardment of seed-axis meristems. This produced clusters of PHB granules in the cytoplasmof the fibre cells [109,113]. Current works in model organisms have shown that PHB granule formation follows a “scaffold model”. Here PHB synthases get linked to a scaffold molecule (DNA and PHA granule associated protein, PhaM) in order to construct the initiation complex for PHA biosynthesis [105]. PHA accumulation(up to 77% of cell dry weight) has been identified in lower algae and bacterial communities extracted via BWWT (Biological Waste Water Treatment). The major categories which accumulate PHA under anaerobic conditions are the phosphorus- accumulating and glycogen-accumulating organisms [105]. Arabidopsis was transformed by the insertion of the phaC1 gene from Pseudomonasaeruginosa, consisting of a peroxisome-targeting sequence from the rape isocitrate lyase. This enhanced the accumulation of medium chain length PHAs in the transformed Arabidopsis [40,113]. Paints,plastics and lubricants have also been synthesized from cyclopropane FAs (CPAs) which act as renewable industrial feedstocks in these manufacturing processes. Less than 1% CPA accumulation occurred by the expression of nine cyclopropane synthases (CPSs) from higherplants in the seeds of fad2 fae1 Arabidopsis. However, 9.1% CPA accumulation was recorded in the seeds, when the Escherichia coli CPS gene was expressed. An impressive 35% CPA accumulation occurred in the transgenic seeds co-expressing Sterculia foetida LPAT (SfLPAT), along with the E. coli CPS [34]. The sn-1 position of phosphatidylcholine is enacted upon by the E. coli CPS. The action of CPS at the sn-2 position of lysophosphatidic acid is promoted by SfLPAT. This results in the high level accumulation of CPA in the coexpressed seeds, due to the enrichment of CPA at both sn-1 and sn-2positions of phosphatidylcholine [34].
Metabolic Engineering of Edible Oils in Plants
Engineering of dietary essential LC-PUFAs in plants
Even today, the major sources of n-3 LC-PUFAs are the fish oils. This is because human desaturases can never add a double bond beyond the ninth carbon atom in the FA chain. LC-PUFAs control a wide range of physiological functions in humans like inflammation, cell growth and the regulation of central nervous system. The deficiency of these FAs results in a range of psychiatricdisorders. The positive effects of these oils on human health have inspired researchers to metabolically engineer the overproduction of these novel LC-PUFAs in plants and even aquatic microalgae. These microalgae belong to the Chromista kingdom, which also consistsof cryptomonads, halophytes and heterokonts [114]. Synthesis and compartmentalization of the n-3 LC-PUFAs have been carried out in Chromista like Nannochloropsis or Isochrysis by mapping the lipid metabolism of the model diatom Phaeodactylum tricornutum. Analysis of genomic data has been performed to develop a coexpressionnetwork [115]. A small number of higher plant specieshave the ability to produce Δ6-desaturated FAs like stearidonic acid (SDA; 18:4 Δ6, 9, 12, 15) and γ-linolenic acid (GLA; 18:3 Δ6, 9, 12) [35]. Approaches are being taken to transfer the biosynthetic pathways of SDA and GLA into commodity crops for cost-effectiveproduction [116]. The rationale behind such engineering is to bypass the flux control step of Δ6-desaturase activity and also the human conversion ratio of dietary SDA to EPA, being 3.3-1.0. This ratio washigher than the 14:1 ratio of dietary ALA (α-linolenic acid) to EPA [115]. Transgenic canola lines were generated by the insertion of Δ6-and Δ12-FA desaturases from commercially raised fungus Mortierella alpine and Δ15-desaturase from canola. As a result, accumulation ofSDA up to 23% of oil by weight was recorded [117].
Metabolic engineering for overproduction of LC-PUFAs along with artemisinic acid (anti-malarial drug precursor) and taxol (anti-cancer agent; spindle depolymerisation inhibitor) have ledto the improvements in the clinical field. Such ω-3 FA products were accumulated by the controlled expression of desaturases and elongases in soybeans and oleaginous yeast [118]. Artemisinin was produced by channelling the flux of the isoprenoid pathway to specificgenes in atremisinin biosynthesis. Taxadiene is the first committed intermediate in taxol biosynthesis, which was isolated by the efficient coupling of the isoprenoid pathway into an E. coli strain [118]. Anartificial pathway producing 26% EPA in leaf TAG was metabolically engineered by introducing a newly identified Δ6-desaturase from the marine microalga Micromonas pusila. This desaturase shared highly conserved motifs with acyl-CoA Δ6-desaturases, according to phylogenetic analyses [119]. Another essential part of the humandiet is tocochromanols (tocopherols and tocotrienols), which act as lipid soluble antioxidants. Genes encoding the limiting enzymes of the tocochromanol pathway were expressed specifically in soybeans.This increased the total tocochromanols up to 15-fold from 320 ng mg-1 in wild type seeds to 4800 ng mg-1 in the best line of seeds [120]. Crossing of transgenic soybean (with high tocochromanol) with transgenic soybean (with high α-tocopherol) could lead to 11-folds increased vitamin E content in the best performing F2seed lines in comparison to the wild type seed [120]. Recently, the overexpression of soyabean GmbZIP123 gene in Arabidopsis enhanced the lipid content in the transgenic seeds. The expression of two sucrose transporter genes (SUC1 and SUC5) and three cell-wall invertase genes (cwINV1, cwINV3, and cwINV6) was also promotedby the GmbZIP123 transgene by binding directly to the promoters of these genes. The increased cell-wall invertase activity and sugar translocation were found in siliques of GmbZIP123 transgenic plants.Higher levels of glucose, fructose and sucrose were also reported in the seeds of GmbZIP123 transgenic plants. All these results indicate to the fact that GmbZIP123 participates in the control of lipidaccumulation in soybean seeds by regulating the sugar transport into seeds from photoautotrophic tissues [121].
Though the implications of the release of FAs from membrane lipids have been established, the knowledge about enzymes and their functions in these processes especially in plants is excruciatingly confined. Patatin-related phospholipase A (pPLA), constituting a major family of acyl-hydrolysing plant enzymes, aids in theaccumulation of TAGs with 20C and 22C long FAs in Arabidopsis. The pPLAIIIΔ out of the four pPLAIIIs (α, β, γ, Δ) decreases the seed oil content when genetically knocked out. The developing embryosalso present a high expression of this pPLA [122]. The FAs are released by pPLAIIIΔ, hydrolyzing phosphatidylcholine (generating lysophosphatidylcholine and free FA) and acyl-CoA. The seeds overexpressing pPLAIIIΔ show no detrimental effects, but there occurs a decrease in their yield. A decrease in the acyl-CoA pool size in pPLAIIIΔ-overexpressed seeds, in comparison to the wildtype, is assumed to be due to the thioesterase activity of pPLAIIIΔ and/or increased phosphatidylcholine turnover and TAG synthesis. Increased pPLAIIIΔ mediated-phosphatidylcholine hydrolysis led to higher synthesis of phosphatidylcholine and increased its turnover. This was due to the improved levels of transcripts like AAPT (aminoalcohol-phosphotransferase) and CCT (choline phosphate: cytidine triphosphate cytidylyl transferase). In the Kennedy pathway,the transcript levels of the glycerolipid producing genes like LPAT, GPAT (glycerol phosphate acetyltransferase), PA (phosphatidate) phosphatase and DGAT (diacylglycerol acetyltransferase) remain high, which may be due to a feed-forward signal relayed by the enhancedsubstrate supplies, as pPLAIIIΔ present in higher amounts elevate the levels of free FAs and lysophosphatidylcholine [122].
Further enhancements in metabolic engineering of γ-linolenic acid and stearidonic acid
About 43% GLA and 5% SDA accumulated in the canola seeds (Brassica napus) by the introduction of Δ6 and Δ12 desaturase genes harboured from the fungus Mortierella alpina. Earlier reports suggest that the expression of Δ6-desaturase from the fungus Pythiumirregulare could produce 40% GLA with a little higher content (i.e., 10%) of SDA in canola seed oil, compared with the gene from the fungus Mortierella alpina [35]. An accumulation of GLA and SDA was observed in tobacco leaves when a Δ6-desaturase gene isolated from cyanobacterium Synechocystis sp., being controlled by a constitutive CaMV35S promoter, was introduced in tobacco leaves [35]. A recent improvement in metabolic engineering shows70% (v/v) content of GLA in the seed oil of a high linolenic acid cultivated species of safflower (Carthamus tinctorius) modified by transformation with Δ6-desaturase from Saprolegnia diclina [123]. Primula vialii utilizes specifically the ALA as a substrate. The linseedlines with SDA (13%) and no GLA were developed by harbouring Δ6-desaturase from Primula vialii. Apart from having the advantage of being devoid of PUFA ω-6 precursor GLA, the accumulated levels of SDA were comparable to those in the commercial plant source Echium spp [124].
The C2 to C5 monocarboxylic acids are generated as a co-product of the Sasol industrial oil-from-coal process in the Fischer-Trpsch reaction water treatment. These short chain monocarboxylic acids act as a potential cheap carbon substrate for the production of GLAby selected transgenic Mucor spp. About 14% GLA among the high accumulated content of neutral lipids (84%) was reported in Mucor circinelloides [125]. Cloning of the Δ6-desaturase gene from Rhizopus stolonifer strain YF6 accumulated 49% of GLA in the recombinantPichia pastoris. The open reading frame (ORF) of the cloned DNA showed high similarity to those of the fungal Δ6-desatuarses with three conserved histidine-rich motifs and HPGG motif [126]. In another research, 25% SDA observed in soybean oil was increased by enzymatic acidolysis [127]. Selective esterification with dodecanol(lauryl alcohol) produces SDA in free FAs. Chemical hydrolysis was done to convert modified soybean oil to the corresponding free FA. Next, the free FA was esterified with dodecanol in presence of 5% Candida rugosa lipase as a biocatalyst. The esterfication reactionwas performed for 4 hours with 1:1 molar dodecanol: free FA ratio which resulted in 58% SDA concentration and 94% recovery [128]. Researchers through experimentations have tried to improve the SDA and GLA content found in the family, Loasaceae and exclusively in the plants under the genus Nasa [129]. Recently, a process usingmathematical modelling of supercritical carbon dioxide extraction for essential oil was developed. Such processes are extremely useful to entirely extract the overproduced lipid from metabolically engineered plant tissues and seeds. This model utilizes the diffusion-controlledregime in the pore and film mass transfer resistances accompanied by the axial dispersion of the mobile phase at dynamic conditions using the Henry’s law [130].
Metabolic engineering of EPA and DHA in plants
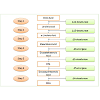
The DHA plays an important role in the developing fetus and is present in the sperm and phospholipids of the brain. Risks of heart diseases, Alzheimer’s disease and retinitis pigmentosa can be reduced if normal levels of DHA are maintained in the body. In plants, theconversion of oleic acid to DHA requires seven steps which are catalysed by seven different enzymes. Linoleic acid is produced from oleic acid by catalysis of Δ12-desaturase. Linoleic acid formsα-linolenic acid by Δ15-desaturase. The latter product is converted to stearidonic acid by Δ6-desaturase. The stearidonic acid is catalysed by Δ6- elongase to eicosatetraenoic acid, which forms EPA by thecatalysis of Δ5-desaturase. The EPA forms docosapentaenoic acid by Δ5-elongase. The docosapentaenoic acid finally produces DHA with the help of Δ4-desaturase [131] (Figure 2).
Figure 2: The seven major steps in the formation of DHA from oleic acid. Each of the reaction steps are catalysed by the mentioned enzymes. The enzymes acting in this pathway for DHA accumulation are either desaturases or elongases. The completion of the third step produces stearidonic acid. The execution of the fifth step heralds the production of EPA. Both stearidonic acid and EPA are important nutrition supplements in the human diet as discussed.
Several marine microalgal strains can accumulate 10-50% oil (w/w) and produce lipids up to 30-70% of the dry weight. During stressful conditions and cell division, these FAs act as sources of energy. High EPA and DHA content have been reported in the strainsfrom genera Phaeodactylum, Nannochloropsis, Thraustochytrium and Schizochytrium. The EPA content was found to be 39% of total FAs in Phaeodactylum tricornutum and Nannochloropsis spp. The heterotrophic growth of the strains like Thraustochytrium and Schizochytrium limacinum accumulated DHA which ranged between 30-40% of total FAs [132]. The acetyl-CoA carboxylase (ACC) wasisolated from the microalga Cyclotella cryptica. This enzyme was then transformed in the diatom Navicula saprophila. Overexpression of thisenzyme alone did not significantly increase the FA content. However, it was the first instance indicating the possibility of overexpressingenzymes in microalgae and diatoms [2]. A natural golden marine algae with a high content of DHA and devoid of other marine oils like menhaden oil was used as a poultry ration supplement. This successfully enhanced the deposition of DHA in the poultry egg yolk [133]. Such egg yolk depositions could be increased by furtheroverproduction of DHA in the algae. Metabolic engineering of the ω-3 FA transgenic trait was performed in P. tricornutum when Δ6-desaturase and Δ5-elongase genes from O. tauri were incorporated into P. tricornutum [134]. No significant increase in total ω-3 FA content occurred in strains expressing Δ6-desaturase. However, the Δ5-elongase expression led to eight fold increase in the DHAconcentration in comparison to the wild type strain. Simultaneous co-expression of OtElo5 and OtD6 accumulated still higher levels of DHA in P. tricornutum. This diatom also contains several active DGAT (diglyceride acyltransferase) genes which catalyses the connection ofan acyl-CoA to the empty sn-3 of DAG [135]. The co-expression of Δ4-desaturase gene with the OtElo5 also resulted in increased levels of DHA in the transgenic diatom [134].
Metabolic engineering to produce essential FAs using algal biofactories has been possible by identifying the genes encoding the enzymes catalyzing FA biosynthesis. Such genes have been found in Ostreococcus tauri, Thalassiosira pseudonana, Phaeodactylumtricornutum and in the model organism Chlamydomonas reinhardtii [132]. Cypthecodinium spp. is another algal source of PUFA which is commercially available and improved methods for overproducingDHA in it are being developed. Recombinant algal biofactories have been used for potential production of EPA and DHA [136]. Chloroplast and nuclear transformation have been successfully carried out on green, red and euglenoid algae. As more information about sequenced plastids, mitochondrial and nucleomorph genomes is gathered, organelle transformation is also on the cards to facilitate metabolic engineering [137]. Such upcomings are based on the complete genome sequences from the red alga Cyanidioschyzon merolae, the diatom Thalassiosira pseudonana, P. tricornutum and the unicellular green alga Ostreococcus tauri [2]. To enhance the EPAbiosynthesis, different gene constructs carrying three to six genes, each under the control of a seed specific promoter was introduced into Arabidopsis. This was done by using floral dip transformation and FA analysis was done in the mature seeds from kanamycinresistantT2 plants [13]. Combination of Δ6- desaturase gene fromO. tauri (OtΔ6), Δ6-fatty acid elongase gene from Physcomitrella patens (PSE1), Δ5-desaturase gene from Thraustochytrium sp., Δ12- desaturase gene from Phytophthora sojae (PsΔ12) and ω3-desaturasefrom P. infestans was prepared to make a five gene construct in order to metabolically engineer the conversion of arachidonic acid (ARA;a C20 ω6 LC-PUFA) to EPA [13]. Microcoleus chthonoplastes Δ15- desaturase or higher plant FAD3 desaturase when incorporated with this gene construct accumulated 11.7% and 12.4% EPA respectively [13].
The EPA-DHA biosynthetic pathway was modified by theincorporation of seven FA biosynthetic genes controlled by seedspecific promoters. The genes were Arabidopsis FAE1, Linum usitatissimum conlinin1 (Cnl1) and conlinin2 (Cnl2) with the truncated Brassica napus napin promoter (FP1) and the tobacco mosaic virus 5’ untranslated enhancer leader sequence, upstream of each fatty acid biosynthesis gene. A constitutively expressed plant selectable marker was added to the gene construct [131]. The genes present in the construct had the ability to convert oleic acid into DHA and these genes were Lachancea kluyveri Δ12-desaturase, Pichia pastoris Δ15-desaturase, Micromonas pusilla Δ6- desaturase, Pyramimonas cordata Δ6- and Δ5-elongases and Pavlova salina Δ5-and Δ4-desaturases. The Δ12- and the Δ15- desaturase genes were abstracted from yeast, while the rest were microalgal in origin. In theleaves of Nicotiana benthamiana, the products of this set of genes resulted in efficient conversion of plant substrate FAs in the leaves to DHA [138]. This set of genes increased the DHA level to 15.1% in the best line. Enrichment of DHA was found at the sn-1/3 position of TAG in Arabidopsis seed oil via 13C Nuclear Magnetic Resonance (NMR) spectroscopy, while insignificant DHA formation was recorded at thesn-2 position of TAG. Total lipid analysis by triple quadrupole LCMS (liquid chromatography-mass spectrometry) showed DHA-18:3- 18:3 (positional distribution not described by the nomenclature) as the most abundant and DHA-18:3-18:2 as the second most abundant DHA-containing TAG species. Low accumulation of tri-DHA TAG was also observed in the total seed oil. In Brassica napus, enrichment at the sn-2 position occurred during the metabolic engineering of ARA [119]. Highest levels consisting of 31% EPA or 25% EPA plus DHA were recorded in metabolically engineered Camelina seed oil[13].
Accumulation of EPA up to 15% of dry cell weight occurs in the oleaginous yeast, Yarrowia lipolytica. Production of 56% EPA and 5% saturated FAs was recorded recently in the metabolically engineered strain of this yeast. These are the highest and least percentages of EPAand saturated FAs respectively recorded till date [139]. The high EPA yields were derived by inactivating the peroxisome biogenesis gene PEX10 [139]. The three genera of thraustochytrids (Thraustochytrium,Schizochytrium and Aurantiochytrium) are the major accumulators of DHA in TAGs during nitrogen starvation [140]. Besides this, an anaerobic polyketide synthase (PKS) pathway is also present inthe heterotrophic thraustochytrid, Schizochytrium for the synthesis of ω-3 LC-PUFAs [115]. The details about this molecule have extensively been discussed in the earlier section. Two genes encoding elongases (tselo1 and tselo2) and a Δ12- desaturase gene involved inDHA synthesis were isolated from Thraustochytrium [141]. The SDA, EPA and DHA are the major FAs of the haptophyta, Pavlova lutheri, containing betaine lipids instead of phosphatidylcholine [115]. A Δ5- elongase encoded by pavELO and a Δ4-desaturase encoded by Pldes1 was found in P. lutheri. These enzymes aided in the elongationof EPA to DHA. Unique substrate specificity was shown by pavELO towards C20 FA, EPA and ARA when expressed in yeast. Formation of docosatetraenoic acid (adrenic acid; 22:4n-6 or 22:4Δ7, 10, 13, 16) and docosapentaenoic acid (22:5n-6) occurred by the desaturationactivity of the enzymatic product of Pldes1 [142,115]. Transgenic Arabidopsis seeds containing cDNAs (encoding acyl-CoA dependent Δ6- and Δ5-desaturases) isolated from the microalga Mantoniella squamata were resurrected. This was done to make a comparative analysis between the conventional lipid linked pathway for LCPUFAsand the acyl-CoA-dependent pathway. The critical analysesshowed that the acyl-CoA pathway avoided the problem of switching the Δ6-desaturase FAs between lipids and acyl-CoA in the seeds [143].
Metabolic engineering of petroselinic acid
Petroselinic acid (18:1Δ6) is an isomer of oleic acid with a cis double bond at the sixth carbon from the carboxyl terminal. This minor structural variance, in comparison to oleic acid, promotespetroselinic acid melting at 33oC, much higher than 12°C as in its isomer. This property of petroselinic acid to be solid at room temperature has been exploited as a source of manufacturing margarine and shortening. This oil is primarily found in the plant species belonging to the families Umbellifereae, Araliaceae andGarryaceae [145]. The pathway for petroselinic acid biosynthesis includes acyl-ACP desaturase, 3-ketoacyl-ACP synthase and acyl- ACP thioesterase. A novel acyl-ACP thioesterase, specifically acting on petroselinoyl-ACP has been found to be the major cause of petroselinic acid in coriander and species of Umbelliferae. Metabolicengineering can be utilised to target this set of three genes to improve the production of this novel plant oil. Single enzyme overproduction might not yield good results. This is because an earlier attempt wasmade by introducing an acyl-ACP desaturase gene from coriander into tobacco. The result showed an increase in petroselinic acid by 5% of the total FAs [146]. Petroselinic acid was also identified inthe seed oil of Geranium sanguineum of Geraniaceae. The seed oil TAG content of this plant primarily consisted of petroselinic acid and also vernolic acid, an epoxy FA [147]. In the industrial sector, lauric acid (12:0) is produced by the oxidation of petroselinic acid byozone. Lauric acid is used in the preparation of soaps and detergents. The oxidation process also yields adipic acid which is used in nylon synthesis. Petroselinic acid is also used in the preparation of cosmetics[40].
Metabolic engineering of peppermint oil in plants
Peppermint oil is one of the most important commercial raw materials used to prepare several mint-flavoured consumer products. The conventional methods in increasing peppermint accumulation have remained demoralizing. This is because peppermint (Mentha piperita) is a sterile hybrid belonging to the Lamiaceae family developed from a cross between water mint (Mentha aquatica) andspearmint (Mentha spicata) and unsuitable for classical breeding. A realistic and quick alternative which has been adopted to increase peppermint oil levels in plants is through metabolic engineering.
One of the prime focuses of metabolic engineering regarding increase in this oil yield is the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway. Overexpression of the 1-deoxy-D-xylulose5-phosphate reductoisomerase (DXR) gene of this pathway increased peppermint oil yield by 44% in comparison to the wild type plants [148,149]. Overexpression of the enzyme (−)-limonene synthase, viz., (−)-LS, was performed in order to enhance the speed of therather slow, rate-limiting first committed step in the p-menthane monoterpene pathway (another target of metabolic engineering). Moderate increase in the oil yield was recorded in this case [150]. Reduction of (+)-menthofuran and its intermediate (+)-pulegoneunder stressful conditions by the expression of (+)-menthofuran synthase (MFS) incorporated in antisense orientation in transgenic lines increased peppermint oil accumulation by 35% [149]. Two-gene combinations with the DXR gene and the antisense version of the MFSincorporated in the transgenic lines resulted in a massive 61% increase of peppermint oil in one of the transgenic lines in comparison to the wild type. This high level of accumulation of the targeted oil was alsoaccompanied by low accumulation of the undesirable side products like (+)-menthofuran and its intermediate (+)-pulegone. Promotion of multi-year field trials in the elite transgenic lines showed a consistent78% increased peppermint oil accumulation compared to the wild type plants. The (+)-limonene aids in the recognition of oil abstracted from transgenic plants during further treatment, processing andcommercial formulations. Elevated levels of (+)-limonene was maintained in the transgenics by overexpressing the (+)-limonene synthase gene [149]. The (+)-Limonene is also a precursor of D-carvone [2-Methyl-5-(1-methylethyenyl)-2-cyclohexenone] generally found in several essential oils and abundant in the seedsof caraway (Carum carvi) and dill. Increase of carvone content via biotechnological developments are being carried out because of the multifarious applications of this monoterpene like fragrance, flavour,antimicrobial effects, biochemical or environmental indicator and relevance in medical applications [151]. Regiospecific biocatalysts have been utilised to enhance limonene biotransformation. Microbial biocatalysts with specific capabilities to hydroxylate the 3-position(isopiperitenol), 6-position (carveol and carvone) and 7-position(perillyl alcohol, perillyl aldehyde and perillic acid) along with 1, 2-position (limonene-1, 2-diol) and the 8-position (α-terpineol) havebeen identified. These findings have aided in the rapid advancement of characterizing plant P-450 limonene hydroxylases and the cloning of the corresponding genes [52]. Increased levels of (+)-limoneneis “generally recognised as safe” (GRAS) by the Federal Drug Administration of the United States and hence can be portrayed as a suitable chemical marker for transgenic plants. The best candidateto serve as a chemical marker gene is a gene encoding (+)-limonene synthase. Due to the fact that commercial essential oil is generally collected from cultivated mint plants by steam distillation, the markerused should be easily detectable by analytical standards, without affecting the organoleptic properties of the oil and also must be present in small amounts. Limonene satisfies all the conditions and therefore it is necessary to overexpress the gene for its production[149].
Recently, a push-pull mechanism postulated higher accumulation and storage of essential oil, via the initiation of leaf glandular trichomes induced by the expression of specific biosynthetic genes[153]. The trichomes are actually specialized anatomical structures in plants with essential functions of oil storage. The first generation mathematical model of peppermint, via a combinatorial approach consisting of computer simulations and experiments showedthat the peppermint oil concentration is highly dependent on the concentrations of MFS and (+)-pulegone reductase with sensitivity towards the density and distribution pattern of the glandular trichomes in leaves [153]. Overexpression of peppermint isopentenyldiphosphate isomerase-encoding specific gene led to 26% increase in peppermint oil in the best yielding transgenic line. Incorporation of the Grand fir (Abies grandis) geranyl diphosphate synthase-encodinggene increased the oil acumulation by 18% over wild types in the best yielding transgenic line [149].
Newer horizons in metabolic engineering in algae andhigher plants: targeting transcription factors (TFs) and TFbinding sites (TFBSs)
As we have already discussed in the preceding sections that oleaginous microalga are potent sources of neutral lipids and are promising biomass feedstocks for production of biofuels. They rarely compete with food crops due to their easy sustainability andcultivability on non-arable lands with non-potable water and waste streams. Evolution in plants deals with several diversifications which can be followed up through critical studies of TF-encoding genes. The in silico proofs extrapolating the relations between TFs and theircognate TFBS motifs on a genome-wide scale are essential to critically modify a biosynthetic pathway using metabolic engineering as a potent tool. Such computational identifications of TFs have been done in green algae like Chlamydomonas reinhardtii and Volvox carteri, inred algae like Galdieria sulphuraria and an Eustigmatophyceae strain N. oceanica CCMP1779 [154]. To explore the genome wide diversity in Nannochloropsis, a phylogenomic approach has been adopted. Comparative study of six genomes of strains of N. oceanica (IMET1 and CCMP531), N. salina (CCMP537), N. gaditana (CCMP526),N. oculata (CCMP525) and N. granulata (CCMP529) unfoldedknowledge about a large pan-genome consisting of more than 38,000 genes [155]. The wide set of shared oleaginous genes identified through this study has presented the proof of diversification and evolution of TF-families and TFBSs in Nannochloropsis. Such findings, alongwith the time-dependent recent generation of large scale, highly reproducible transcript profiles from the strain IMET1, using mRNA-Seq under N-replete (N+) and N-depleted (N-) conditions, has raised the scope to target specific TFs and their binding sites for increase in the metabolic accumulation of the desired products inthe microalgae [154,156]. The mRNA-Seq studies identified 125 TFs, out of which 30 TFs have the potential to control lipid biosynthetic pathways. The major TF-families characterising lipid metabolism areMYB-related (5), NF-YC (5), AP2 (4) and C3H (4). Three of these TFs are the orthologs of WRI1 (WRINKLED1), while gene expression correlation analyses of mRNA-Seq data helped in the identification of other TFs. The WRI1 family of TFs are the major regulators of oil synthesis in Arabidopsis seeds. This TF-family belongs to the AP2/EREBP family of TFs, sharing one or two copies of AP2 DNA-binding domain(s) [154].
A phylogenetic relation is sustained among the microalgal species. Similar TF-family profiles also indicate the possibility of co-evolution of the species. The MERCED algorithm was utilised to identify the TFBSs in the species of Nannochloropsis. About 68TFBSs were found to be “most conserved”, out of which 11 had some photosynthetic or lipid accumulation links [157]. The regulatory network that needs to be modified to enhance biofuel production in Nannochloropsis was made clearer by a comparative analysis between the TFs-TFBS interactions of IMET1 and those of the reference plant using TRANSFAC (TRANScription FACtor database). The results indicated the presence of 78 TF-TFBS interaction pairs consisting of 34 TFs, 30 TFBSs and 950 target genes. Out of the 34identified TFs, 11 showed involvements in the TAG biosynthesis pathway [158]. Nannochloropsis, green algae and red algae have been distinguished from higher plants by the TFs MYB, MYB-related, bZIP, C2H2 and C3H. Nannochloropsis, green algae and red algaecan be separated from each other by the TFs bHLH, NAC, ERF, C3H and WRKY. These identifications of TF-families were done through Principal Component Analysis (PCA) [154]. The WRKY family is one of the largest and most important TF-families in plants due totheir diverse abilities to specify, transduce and control the varied signaling pathways. The ChIP-Seq (Chromatin Immunoprecipitation Sequencing) ushered by in silico methods have been suggested to further improve the knowledge for metabolic engineering of the TAGbiosynthetic pathway in microalgae for biofuel production [154](Figure 3).
Figure 3: Modulation of the transcription factors (TFs) to enhance lipid production in plants. The targets TFs in higher plants and the algal model, Nannochloropsis have been depicted. These groups of TFs are thought to contain some phylogenetic significance in the process of lipid accumulation in the tissues in due courseof evolution.
The TF ORCA2 stimulated terpenoid indole alkaloid (TIA) biosynthesis in higher plants like Catharanthus roseus. During seed development, storage lipid synthesis and accumulation is affected by SebHLH (a bHLH family TF of Sesamum indicum). Overexpressing Dof-type (DNA binding with one finger) TF genes, GmDof4 and GmDof11 of Glycine max resulted in an increase in the lipid contentin transgenic Arabidopsis seeds [2]. The unicellular green alga Chlamydomonas reinhardtii, moss Physcomitrella patens, pteridophyte Selaginella moellendorffii, gymnosperm Pinustaeda, dicotyledons A. thaliana and monocotyledons Oryza sativa and Hordeum vulgarewere all found to possess Dof-type TF-family sequences [159]. Lipid homeostasis in mammals is regulated by sterol regulatory element binding protein (SREBP). Such SREBPs with high conservative sequences have also been found in plants and microorganisms. The SREBP (Sre1p) in fission yeast Schizosaccharomyces pombe targeted the oxygen-requiring biosynthetic pathways for ergosterol, heme, sphingolipid and ubiquinone. The Sre1p binds directly at the target gene promoters [160,2].
Conclusion
Metabolic engineering has provided the vision of converting plants into chemical factories of lipid synthesis by moulding the structure of FA profiles in plants according to human benefit. Thiswill facilitate abundant and economically feasible production of FAs, benefitting the industrial sectors along with human health. Isolation of the necessary target genes has been a major bottleneck for theabsolute success of this technology. Another major field that is being excavated is the adaptive evolution of seed TAGs. Such researches have been performed in Arabidopsis. Seed TAGs have shown latitudinal cline and plasticity in adaptive responses to an increase in temperature. Thus, utilising the genetic tools present to manipulatethe adaptive responses in Arabidopsis, a model system can be generated out of it to study seed oil evolution and the breeding of seed oil crops [161]. Appearances of newer and modern genetic tools and techniques have improved the probabilities of formulating thedesign of newer enzymes. The overexpression of these new enzymes with diverse structures and biochemical complexities can inculcate higher accumulation of target products in the plant system in future.
Acknowledgements
Financial support from Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India through the research grant (SR/FT/LS-65/2010) to Dr. Aryadeep Roychoudhury is gratefully acknowledged.
References
- Burgal J, Shockey J, Lu C, Dyer J, Larson T, et al. (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6: 819-831.
- Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141: 31-41.
- Bornke F, Broer I (2010) Tailoring plant metabolism for the production of novel polymers and platform chemicals. Curr Opin Plant Biol 13: 354-362.
- Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18: 134-141.
- Abdel-Ghany SE, Day I, Heuberger AL, Broeckling CD, Reddy ASN (2013) Metabolic engineering of Arabidopsis for butanetriol production using bacterial genes. Metab Eng 20: 109-120..
- Poirier Y (2001) Production of polyesters in transgenic plants. Adv Biochem Eng Biotechnol 71: 209-240.
- Yue AQ, Sun XP, Li RZ (2007) Metabolic engineering of edible plant oils (article in Chinese). Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 33: 489-498.
- Napier JA, Haslam RP, Beaudoin F, Cahoon EB (2014) Understanding and manipulating plant lipid composition: Metabolic engineering leads the way. Curr Opin Plant Biol 19: 68-75.
- Cressey D (2009) Aquaculture: future fish. Nature 458: 398-400.
- Saravanan P, Davidson NC, Schmidt EB, Calder PC (2010) Cardiovascular effects of marine omega-3 fatty acids. Lancet 376: 540-550.
- Domergue F, Abbadi A, Heinz E (2005) Relief for fish stocks: oceanic fatty acids in transgenic oil seeds. Trends Plant Sci 10: 112-116.
- Dyer JM, Stymne S, Green AG, Carlsson AS (2008) High-value oils from plants. Plant J 54: 640-655.
- Ruiz-Lopez N, Haslam RP, Usher SL, Napier JA, Sayanova O (2013) Reconstitution of EPA and DHA biosynthesis in Arabidopsis: Iterative metabolic engineering for the synthesis of n−3 LC-PUFAs in transgenic plants. Metab Eng 17: 30-41.
- Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, et al. (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16: 2734-2748.
- Napier JA, Sayanova O, Qi B, Lazarus CM (2004) Progress toward the production of long-chain polyunsaturated fatty acids in transgenic plants. Lipids 39: 1067-1075.
- Riederer M, Muller C (2006) Biology of the Plant Cuticle. Oxford: Blackwell.
- Jetter R, Kunst L (2008) Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J 54: 670-683.
- Heilmann M, Iven T, Ahmann K, Hornung E, Stymne S, et al. (2012) Production of wax esters in plant seed oils by oleosomal cotargeting of biosynthetic enzymes. J Lipid Res 53: 2153-2161.
- Khor YP, Koh SP, Long K, Long S, Ahmad SZ, et al. (2014) A comparative study of the physicochemical properties of a virgin coconut oil emulsion and commercial food supplement emulsions. Molecules 19: 9187-9202.
- Broun P, Somerville C (2001) Progress in plant metabolic engineering. Proc Natl Acad Sci USA 98: 8925-8927.
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, et al. (2013) Acyl-lipid metabolism. Arabidopsis Book 11: e0161.
- Pojer F, Noel JP, Larson E, Bowman M, Stephane R (2008) Metabolic engineering of lipid metabolism by improving fatty acid binding and transport. International Publication No.: WO 2008/008467 A2, International Patent Classification: A01H 5/00 (2006.01).
- Beer LL, Boyd ES, Peters JW, Posewitz MC (2009) Engineering algae for biohydrogen and biofuel production. Curr Opin Biotechnol 20: 264-271.
- Rossi M, Amaretti A, Raimondi S, Leonardi A (2011) Getting lipids for biodiesel production from oleaginous fungi. Biodiesel - Feedstocks and Processing Technologies, Dr. Margarita Stoytcheva (Ed.) ISBN: 978-953-307-713-0: 71-92.
- Chapman KD, Dyer JM, Mullen RT (2012) Biogenesis and functions of lipid droplets in plants: thematic review series: lipid droplet synthesis and metabolism: from yeast to man. J Lipid Res 53: 215-226.
- Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep 27: 273-278.
- Nguyen HT, Silva JE, Podicheti R, Macrander J, Yang W, et al. (2013) Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant Biotechnol J 11: 759-769.
- Naim F, Nakasugi K, Crowhurst RN, Hilaro E, Zwart AB, et al. (2012) Advanced engineering of lipid metabolism in Nicotiana benthamiana using a draft genome and the V2 viral silencing-suppressor protein. PLoS ONE 7:e52717. Indian Forester 131.
- Bates PD, Stymne S, Ohlrogge J (2013) Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol 16: 358-364.
- van Erp H, Bates PD, Burgal J, Shockey J, Browse J (2011) Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol 155: 683-693.
- Xu R, Yang T, Wang R, Liu A (2014) Characterisation of DGAT1 and DGAT2 from Jatropha curcas and their functions in storage lipid biosynthesis. Funct Plant Biol 41: 321-329.
- Hu Z, Ren Z, Lu C (2012) The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol 158: 1944-1954.
- Yu W, Ansari W, Schoepp NG, Hannon MJ, Mayfield SP, et al. (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Fact 10: 91.
- Yu XH, Prakash RR, Sweet M, Shanklin J (2014) Coexpressing Escherichia coli cyclopropane synthase with Sterculia foetida lysophosphatidic acid acyltransferase enhances cyclopropane fatty acid accumulation. Plant Physiol 164: 455-465.
- Haslam RP, Ruiz-Lopez N, Eastmond P, Moloney M, Sayanova O, et al. (2013) The modification of plant oil composition via metabolic engineering — better nutrition by design. Plant Biotechnol J 11:157-168.
- Li X, van Loo EN, Gruber J, Fan J, Guan R, et al. (2012) Development of ultra-high erucic acid oil in the industrial oil crop Crambe abyssinica. Plant Biotechnol J 10: 862-870.
- Guan R, Lager I, Li X, Stymne S, Zhu LH (2013) Bottlenecks in erucic acid accumulation in genetically engineered ultrahigh erucic acid Crambe abyssinica. Plant Biotechnol J 12: 193-203.
- Pham A, Lee J, Shannon J, Bilyeu K (2010) Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol 10: 195.
- Jadhav A, Katavic V, Marillia EF, Michael Giblin E, Barton DL, et al. (2005) Increased levels of erucic acid in Brassica carinata by co-suppression and antisense repression of the endogenous FAD2 gene. Metab Eng 7: 215-220.
- Slater A, Scott N, Fowler M (2008) Plant Biotechnology: the genetic manipulation of plants. Second edition © Oxford University Press.
- Christie WW (2013) Fatty acids: hydroxyl, epoxy, furanoid, methoxyl, oxo-structures, occurrence and biochemistry. © lipidlibrary.aocs.org.
- Napier JA (2007) The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol 58: 295-319.
- Nguyen HT, Mishra G, Whittle E, Pidkowich MS, Bevan SA, et al. (2010) Metabolic engineering of seeds can achieve levels of ω-7 fatty acids comparable with the highest levels found in natural plant sources. Plant Physiol 154: 1897-1904.
- Whittle E, Shanklin J (2001) Engineering delta 9-16:0-acyl carrier protein (ACP) desaturase specificity based on combinatorial saturation mutagenesis and logical redesign of the castor delta 9-18:0-ACP desaturase. J Biol Chem 276: 21500-21505.
- Kumar AN, Joshi G, Mohan Ram HY (2012) Sandalwood: history, uses, present status and the future. Curr Sci 103: 1408-1416.
- Diaz-Chavez ML, Moniodis J, Madilao LL, Jancsik S, et al. (2013) Biosynthesis of Sandalwood oil: Santalum album CYP76F cytochromes P450 produce santalols and bergamotol. PloS ONE 8: e75053.
- Wang Q, Hillwig ML, Okada K, Yamazaki K, Wu Y, et al. (2012) Characterization of CYP76M5-8 Indicates metabolic plasticity within a plant biosynthetic gene cluster. J Biol Chem 287: 6159-6168.
- Jones CG, Keeling CI, Ghisalberti EL, Barbour EL, Plummer JA, et al. (2008) Isolation of cDNAs and functional characterization of two multi-product terpene synthase enzymes from sandalwood, Santalum album L. Arch Biochem Biophys 477: 121-130.
- Jones CG, Moniodis J, Zulak KG, Scaffidi A, Plummer JA, et al. (2011) Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J Biol Chem 286: 17445-17454.
- Misra BB, Dey S (2013) Developmental variations in sesquiterpenoid biosynthesis in East Indian sandalwood tree (Santalum album L.). Trees 27: 1071-1086.
- Kobayashi M, Saitoh M, Ishida K, Yachi H (2005) Viscosity properties and molecular structure of lube base oil prepared from Fischer-Tropsch waxes. J Jpn Pet Inst 48: 365-372.
- FAOSTAT (2008) ProdSTAT: Crops. Rome: Food and Agriculture Organization of the United Nations.
- Botti C, Prat L, Palzkill D, Canaves L (1998) Evaluation of jojoba clones grown under water and salinity stresses in Chile. Ind Crop Prod 9: 39-45.
- Lardizabal KD, Metz JG, Sakamoto T, Hutton WC, Pollard MR, et al. (2000) Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol 122: 645-655.
- Metz JG, Pollard MR, Anderson L, Hayes TR, Lassner MW (2000) Purification of a jojoba embryo fatty acyl-coenzyme A reductase and expression of its cDNA in high erucic acid rapeseed. Plant Physiol 122: 635-644.
- Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, et al. (2006) CER4 encodes an alcohol-forming fatty acyl-CoA reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 142: 866-877.
- Zheng H, Rowland O, Kunst L (2005) Disruptions of the Arabidopsis enoyl-CoA reductase gene reveals an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 17: 1467-1481.
- Carlsson AS (2006) Production of Wax Esters in Crambe. EpoBIOReport.
- Lai C, Kunst L, Jetter R (2007) Composition of alkyl esters in the cuticular wax on inflorescence stems of Arabidopsis thaliana cer mutants. Plant J 50: 189-196.
- von Wettstein-Knowles P (2007) Analyses of barley spike mutant waxes identify alkenes, cyclopropanes and internally branched alkanes with dominating isomers at carbon 9. Plant J 49: 250-264.
- Jaworski J, Cahoon EB (2003) Industrial oils from transgenic plants. Curr Opin Plant Biol 6: 178-184.
- Bernard A, Joubes J (2013) Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Prog Lipid Res 52: 110-129.
- Jakobsson A, Westerberg R, Jacobsson, A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45: 237-249.
- Yen CL, Brown CH, IV, Monetti M, Farese RV Jr (2005) A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J Lipid Res 46: 2388-2397.
- Haribal M, Dhondt AA., Rosane D, Rodriguez E (2005) Chemistry of preen gland secretions of passerines: different pathways to same goal? Why? Chemoecology 15: 251-260.
- Ishige T, Tani A, Sakai Y, Kato N (2003) Wax ester production by bacteria. Curr Opin Microbiol 6: 244-250.
- Ishige T, Tani A, Takabe K, Kawasaki K, et al. (2002) Wax ester production from n-alkanes by Acinetobacter sp. strain M-1: ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl Environ Microbiol 68: 1192-1195.
- Cheng JB, Russell DW (2004a) Mammalian wax biosynthesis: I. Identification of two fatty acyl-CoA reductases with different substrate specificities and tissue distributions. J Biol Chem 279: 37789-37797.
- Cheng JB, Russell DW (2004b) Mammalian wax biosynthesis: II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J Biol Chem 279: 37798-37807.
- Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, et al. (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18: 2294-2313.
- Bird D, Beisson F, Brigham A, Shin J, Greer S, et al. (2007) Characterization of Arabidopsis WBC11/ABCG11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52: 485-498.
- Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, et al. (2007) The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 145: 1345-1360.
- Doran-Peterson J, Cook DM, Brandon SK (2008) Microbial conversion of sugars from plant biomass to lactic acid or ethanol. Plant J 54: 582-592.
- Pauly M, Keegstra K (2008) Cell wall carbohydrates and their modifications as a resource for biofuels. Plant J 54: 559-568.
- Lin W, Oliver DJ (2008) Role of triacylglycerols in leaves. Plant Sci 175: 233-237.
- Kelly AA, van Erp H, Quettier AL, Shaw E, Menard G, et al. (2013) The Sugar-Dependent1 lipase limits triacylglycerols accumulation in vegetative tissues of Arabidopsis. Plant Physiol 162: 1282-1289.
- Vanhercke T, El Tahchy A, Shrestha P, Zhou XR, Singh SP, Petrie JR (2013) Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett 587: 364-369.
- Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, et al. (2010) Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol J 8: 277-287.
- Santos Mendoza M, Dubreucq B, Miquel M, Caboche M, Lepiniec L (2005) LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett 579: 4666-4670.
- Sanjaya, Miller R, Durrett TP, Kosma DK, Lydic TA, et al. (2013) Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell 25: 677-693.
- Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, et al. (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J 7: 694-703.
- James CN, Horn PJ, Case CR, Gidda SK, Zhang D, et al. (2010) Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman- like lipid droplet accumulation in plants. Proc Natl Acad Sci USA 107: 17833-17838.
- Vanhercke T, El Tahchy A, Liu Q, Zhou XR, Shrestha P, et al. (2014) Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol J 12: 231-239.
- Trentacoste EM, Shrestha RP, Smith SR, Gle C, Hidelbrand M, et al. (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci USA 110: 19748-19753.
- Matthijs M, Fabris M, Baart GJE, Goossens A, Vyverman W (2012) Enhancing lipid production of Phaeodactylum tricornutum through metabolic engineering. XXII International Diatom Symposium.
- León-Bañares R, González-Ballester D, Galván A, Fernández E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22: 45-52.
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, et al. (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621-639.
- Neupert J, Karcher D, Bock R (2008) Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J 57: 1140-1150.
- Shao N, Bock R (2008) A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Curr Genet 53: 381-388.
- Zhao T, Wang W, Bai X, Qi Y (2009) Gene silencing by artificial microRNAs in Chlamydomonas. Plant J 58: 157-164.
- Li X, Hu HY, Zhang (2011) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102: 3098-3102.
- Azócar L, Ciudad G, Heipieper H J, Navia R (2010) Biotechnological processes for biodiesel production using alternative oils. Appl Microbiol Biotechnol 88: 621-636.
- Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90: 1219-1227.
- Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51: 1-51.
- Mlickova K, Luo Y, d´Andrea S, Pec P, Nicaud JM, et al. (2004a) Acyl-CoA oxidase, a key step for lipid accumulation in the yeast Yarrowia lipolytica. J Mol Catal B Enzym 28: 81-85.
- MlÃckova K, Roux E, Athenstaedt K, d'Andrea S, Daum G, et al. (2004b) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl Environ Microbiol 70: 3918-3924.
- Chi Z, Chi Z, Zhang T, Liu GL, Yue L (2009) Inulinase expressing microorganisms and applications of inulinases. Appl Microbiol Biotechnol 82: 211-220.
- Blazeck J, Hill A, Liu L, Knight R, Miller J, et al. (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5: doi: 10.1038/ncomms4131.
- Sheik AR, Muller EEL, Wilmes P (2014) A hundred years of activated sludge: time for a rethink. Front Microbiol 5:47.
- Park HJ, Heo HS, Park YK, Yim JH, Jeon JK, et al. (2010) Clean bio-oil production from fast pyrolysis of sewage sludge: effects of reaction conditions and metal oxide catalysts. Bioresour Technol 101: S83-S85.
- Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19: 257-275..
- Mahapatra DM, Chanakya HN, Ramachandra TV (2013) Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J Appl Phycol 25: 855-865.
- D’Oca MGM, Viegas CV, Lemoes JS, Miyasaki EK, Maron-Villarreyes JA, et al. (2011) Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioenerg 35: 1533-1538.
- Knothe G (2008) ‘Designer’ biodiesel: optimizing fatty ester composition to improve fuel properties. Energy & Fuels 22: 1358-1364.
- Widenhorn-Müller K, Schwanda S, Scholz E, Spitzer M, Bode H (2014) Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prost Leuk Ess Fatty Acids 91: 49-60.
- Craggs RJ, Heubeck S, Lundquist TJ, Benemann JR (2011) Algal biofuels from wastewater treatment high rate algal ponds. Water Sci Technol 63: 660-665.
- Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A (2013) Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int J Biomater 2013: 752821.
- Reddy CS, Ghai R, Rashmi, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87: 137-146.
- Conrad U (2005) Polymers from plants to develop biodegradable plastics. Trends Plant Sci 10: 511-512.
- Bohmert K, Balbo I, Steinbuchel A, Tischendorf G, Willmitzer L (2002) Constitutive expression of the β-ketothiolase gene in transgenic plants. A major obstacle for obtaining polyhydroxybutyrate-producing plants. Plant Physiol 128: 1282-1290.
- Potter M, Muller H, Steinbuchel (2005) An influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 151: 825-833
- Schnell JA, Treyvaud-Amiguet V, Arnason JT, Johnson DA (2012) Expression of polyhydroxybutyric acid as a model for metabolic engineering of soybean seed coats. Transgenic Res 21: 895-899.
- Scheller J, Conrad U (2005) Plant-based material protein and biodegradable plastic. Curr Opin Plant Biol 8: 188-196.
- Cavalier-Smith T (2010) Kingdoms protozoa and chromista and the eozoan root of the eukaryotic tree. Biol Lett 6: 342-345.
- Muhlroth A, Li K, Rokke G, Winge P, Olsen Y, et al. (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11: 4662-4697.
- Flider FJ (2005) GLA: uses and new sources. Inform 16: 279-282.
- Ursin VM (2003) Modification of plant lipids for human health: Development of functional land-based omega-3 fatty acids. J Nutr 133: 4271-4274.
- Ye VM, Bhatia SK (2012) Metabolic engineering for the production of clinically important molecules: Omega-3 fatty acids, artemisinin, and taxol. Biotechnol J 7: 20-33.
- Petrie JR, Shrestha P, Mansour MP, Nichols PD, Liu Q, et al. (2010) Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Delta6-desaturase with omega3-preference from the marine microalga Micromonas pusilla. Metab Eng 12: 233-240.
- Karunanandaa B, Qi Q, Hao M, Baszis SR, Jensen PK, et al. (2005) Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab Eng 7: 384-400.
- Song QX, Li QT, Liu YF, Zhang FX, Ma B, et al. (2013) Soybean GmbZIP123 gene enhances lipid content in the seeds of transgenic Arabidopsis plants. J Exp Bot 64: 4329-4341.
- Li M, Bahn SC, Fan C, Li J, Phan T, et al. (2013) Patatin-related phospholipase pPLAIIIΔ increases seed oil content with long-chain fatty acids in Arabidopsis. Plant Physiol 162: 39-51.
- Nykiforuk CL, Shewmaker C, Harry I, Yurchenko OP, Zhang M, et al. (2012) High level accumulation of gamma linolenic acid (C18:3Δ6.9,12 cis) in transgenic safflower (Carthamus tinctorius) seeds. Transgenic Res 21: 367-381.
- Ruiz-Lopez N, Haslam RP, Venegas-Caleron M, Larson TR, Graham IA, et al. (2009) The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of ‘heart-healthy’ omega-3 fatty acids. Plant Biotechnol J 7: 704-716.
- du Preez JC, Immelman M, Kilian SG (1996) The utilization of short-chain monocarboxylic acids as carbon sources for the production of gamma-linolenic acid by Mucor strains in fed-batch culture. World J Microbiol Biotechnol 12: 68-72.
- Wan X, Zhang Y, Wang P, Jiang M (2011) Molecular cloning and expression analysis of a delta 6-fatty acid desaturase gene from Rhizopus stolonifer strain YF6 which can accumulate high levels of gamma-linolenic acid. J Microbiol 49: 151-154.
- Ifeduba EA, Akoh CC (2013) Chemoenzymatic Method for producing stearidonic acid concentrates from stearidonic acid soybean oil. J Amer Oil Chem Soc 90: 1011-1022.
- Vazquez L, Kleiner L, Akoh CC (2012) Concentration of stearidonic acid in free fatty acids form from modified soybean oil by selective esterification with dodecanol. J Amer Oil Chem Soc 89: 1655-1662.
- Aitzetmuller K, Bruhl L, Weigend M (2004) Seed oil fatty acids of loasaceae—A new source of γ-linolenic and stearidonic acids. J American Oil Chemist’s Society 81: 259-263.
- Ghoreishi SM, Bataghva E (2014) Supercritical extraction of essential oil from Echium amoenum seed: Experimental, modeling and genetic algorithm parameter estimation. Korean J Chem Eng 2014: 1-9.
- Petrie JR, Shrestha P, Zhou XR, Mansour MP, Liu Q, et al. (2012) Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE 7: e49165.
- Adarme-Vega TC, Lim DKY, Timmins M, Vernen F, Li Y, et al. (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact 11: 96.
- Herber SM, Van Elswyk ME (1996) Dietary marine algae promotes efficient deposition of n-3 fatty acids for the production of enriched shell eggs. Poult Sci 75: 1501-1507.
- Hamilton ML, Haslam RP, Napier JA, Sayanova O (2014) Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega3 long chain poly unsaturated fatty acids. Metab Eng 22: 3-9.
- Gong Y, Zhang J, Guo X, Wan X, Liang Z, et al. (2013) Identification and characterization of PtDGAT2B an acyltransferase of the DGAT2 acyl-Coenzyme A: diacyl glycerol acyltransferase family in the diatom Phaeodactylum tricornutum. FEBS Lett 587: 481-487.
- Li YT, Li MT, Fu CH, Zhou PP, Liu JM, et al. (2009) Improvement of arachidonic acid and eicosapentaenoic acid production by increasing the copy number of the genes encoding fatty acid desaturase and elongase into Pichia pastoris. Biotechnol Lett 31: 1011-1017.
- Walker TL, Collet C, Purton S (2005) Algal transgenics in the genomic era. J Phycol 41: 1077-1093.
- Petrie JR, Singh SP (2011) Expanding the docosahexaenoic acid food web for sustainable production: engineering lower plant pathways into higher plants. AoB Plants 2011: plr011.
- Xue Z, Sharpe PL, Hong SP, Yadav NS, Xie D, et al. (2013) Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat Biotechnol 31: 734-740.
- Martins DA, Custodio L, Barreira L, Pereira H, Ben-Hamadou R, et al. (2013) Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs 11: 2259-2281.
- Ohara J, Sakaguchi K, Okita Y, Okino N, Ito M (2013) Two fatty acid elongases possessing C18-Δ6/C18-Δ9/C20-Δ5 or C16-Δ9 elongase activity in Thraustochytrium sp. ATCC 26185. Mar Biotechnol 15: 476-486.
- Pereira SL, Leonard AE, Huang YS, Chuang LT, Mukerji P (2004) Identification of two novel microalgal enzymes involved in the conversion of the omega3-fatty acid, eicosapentaenoic acid, into docosahexaenoic acid. Biochem J 384: 357-366.
- Hoffman M, Wagner M, Abbadi A, Fulda M, Feussner I (2008) Metabolic engineering of omega-3 very long chain polyunsaturated fatty acid production by an exclusively acyl-CoA-dependent pathway. J Biol Chem 283: 22352-22362.
- Ruiz-Lopez N, Sayanova O, Napier JA, Haslam RP (2012) Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J Exp Bot 63: 2397-2410.
- Dyer JM, Mullen RT (2005) Development and potential of genetically engineered oilseeds. Seed Sci Res 15: 255-267.
- Ohlrogge JB (1994) Design of new plant products: Engineering of fatty acid metabolism. Plant Physiol 104: 821-826.
- Tsevegsuren N, Aitzetmuller K, Vosmann K (2004) Geranium sanguineum (Geraniaceae) seed oil: a new source of petroselinic and vernolic acid. Lipids 39: 571-576.
- Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98: 8915-8920.
- Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM, et al. (2011) Improving peppermint essential oil yield and composition by metabolic engineering. Proc Natl Acad Sci USA 108: 16944-16949.
- Diemer F, Caissard JC, Moja S, Calchat JC, Jullien F (2001) Altered monoterpene composition in transgenic mint following the introduction of 4S-limonene synthase. Plant Physiol Biochem 39: 603-614.
- de Carvalho CCCR, da Fonseca MMR (2006) Carvone: why and how should one bother to produce this terpene? Food Chem 95: 413-422.
- Duetz WA, Bouwmeester H, van Beilen JB, Witholt B (2003) Biotransformation of limonene by bacteria, fungi, yeasts and plants. Appl Microbiol Biotechnol 61: 269-277.
- Rios-Estepa R, Lange I, Lee JM, Lange BM (2010) Mathematical modeling-guided evaluation of biochemical, developmental, environmental and genotypic determinants of essential oil composition and yield in peppermint leaves. Plant Physiol 152: 2105-2119.
- Hu J, Wang D, Li WJ, Jing G, Ning K, et al. (2014) Genome-wide identification of transcription factors and transcription-factor binding sites in oleaginous microalgae Nannochloropsis. Nature Scientific Report 4: 5454.
- Wang D, Ning K, Li J, Hu J, Han D, et al. (2014) Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLoS Genet 10: e1004094.
- Li J, Han D, Wang D, Ning K, Jia J, et al. (2014) Choreography of transcriptomes and lipidomes of Nannochloropis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 26: 1214-1218.
- Ding J, Li X, Hu H (2012) Systematic prediction of cis-regulatory elements in the Chlamydomonas reinhardtii genome using comparative genomics. Plant Physiol 160: 613-623.
- Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, et al. (2006) TRANSFACH and its module TRANSCompelH: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108-D110.
- Moreno-Risueno MA, Martinez M, Vicente-Carbajosa J, Carbonero P (2007) The family of DOF transcription factors: from green unicellular algae to vascular plants. Mol Gen Genomics 277: 379-390.
- Todd BL, Stewart EV, Burg JS, Hughes AL, Espenshade PJ (2006) Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol Cell Biol 26: 2817-2831.
- Sanyal A, Linder CR (2013) Plasticity and constraints on fatty acid composition in the phospholipids and triacylglycerols of Arabidopsis accessions grown at different temperatures. BMC Plant Biol 13: 63.