Anti-Proliferative Evaluation of Benzo [6,7] Cyclohepta [1,2-b] Pyridine Derivatives in Addition to Anti-Inflammatory Activity
Yasodakrishna Sajja1, Sowjanya Polepalli2, Nishant Jain2 and Lingaiah Nagarapu1*
1Organic Chemistry Division-II (CPC), CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad, India
2Centre for Chemical Biology, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad, India
Corresponding author: Lingaiah Nagarapu, Organic Chemistry Division-II (CPC), CSIR-Indian Institute of ChemicalTechnology, Tarnaka, Hyderabad-500007, India
Citation: Yasodakrishna S, Sowjanya P, Nishant J, Lingaiah N. Anti-Proliferative Evaluation of Benzo [6,7] Cyclohepta [1,2-b] Pyridine Derivatives in 02 Addition to Anti-Inflammatory Activity. J Cancer Res Molecul Med. 2016;3(1): 104.
Copyright © 2016 Yasodakrishna Sajja, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cancer Research and Molecular Medicine | Volume: 3, Issue: 1
Submission: Submission: 26/08/2016; Accepted: 04/11/2016; Published: 08/11/2016
Abstract
A variety of benzo [6,7] cyclohepta [1,2-b] pyridine derivatives have been evaluated for their in vitro anti-proliferative activity against four cancer cell lines such as HeLa (cervical), MIAPACA (pancreatic), MDA-MB-231 (breast) and IMR32 (neuroblastoma). Among tested compounds, a exhibited potent antiproliferative activity against IMR32 with GI50 value less than 0.01 μM and compounds d, l and n exhibited promising anti-proliferative activity against IMR32 with GI50 values 0.1, 0.21 and 0.21 μM, respectively. This is the first report on in vitro anti-proliferative evaluation of benzo [6,7] cyclohepta [1,2-b] pyridine derivatives in addition to anti-inflammatory activity.
Keywords: Benzosuberone; Benzo [6,7] cyclohepta [1,2-b] pyridines; Anti-proliferative; Cell lines; Doxorubicin; Paclitaxel
Introduction
Benzosuberone motif has remarkable biological activities such as anti-inflammatory, anti-pyretic, anti-ulcer, CNS-depressant, CNS stimulant and anticonvulsant activities. And some of the derivativesare also known for anti-tumor activity in murine p338 cell line tests[1]. In addition these derivatives widely used in pharmaceutical applications, such as tricyclic antidepressants containingdibenzosuberone moieties mostly effect the autonomic and central nervous systems, and traditional anti-depressants, like amitriptyline [2], imipramine [3] and noxiptiline [4] which continue to be used as first-line drugs in treating depressive disorders.
Pyridyl compounds are of interest to organic chemists in recent years owing to their wide spectrum of physiological activity [5-10]. The condensed derivatives of pyridines play significant role in bioactive molecules, especially in the form of benzo [5,6] cyclohepta [1,2-b] pyridines which are structural analogues to benzosuberone. The benzo [5,6] cyclohepta [1,2-b] pyridine is an important core biologically active compound with diverse biological activities, such as antihistamine as well as antitumor and antiinflammatory activities [11-17]. It is a highly potent pharmacophore and widely used in drug molecular design. Because of the important aforementioned properties of benzocyclohepta [1,2-b] pyridines derivatives, preparation of this heterocyclic nucleus has gained great importance in organic synthesis.
Recently, we have published [18] synthesis and anti-inflammatory activity of benzo [6,7] cyclohepta [1,2-b] pyridines, in the present manuscript, presenting in vitro anti-proliferative activity of benzo [6,7] cyclohepta [1,2-b] pyridines against four cancer cell lines such as HeLa (cervical), MIAPACA (pancreatic), MDA-MB-231 (breast) and IMR32 (neuroblastoma) and a SRB cell proliferation assay to estimate viability or growth in addition to anti-inflammatory activity in continuation to our ongoing research activities [9-27], to discover and develop tumor growth inhibitors and apoptotic inducers as potential new anti-cancer agents.
Effects of the Compounds on the Viability of Human Cancer Cells
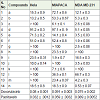
All the synthesized compounds of benzo [6,7] cyclohepta [1,2-b] pyridine derivatives (a-n) were screened for their in vitro antiproliferative activity against four cancer cell lines such as HeLa(cervical), MIAPACA (pancreatic), MDA-MB-231 (breast) and IMR32 (neuroblastoma) summarized in Table 1.
The compounds were picked for an advanced assay against these four human cancer cell lines at five concentrations (0.01, 0.1, 1, 10, 100 μM). GI50 (growth inhibitory activity) was calculated and these values corresponded to the concentration of the compound causing 50% decrease in the net cell growth as compared with the standard drugs, doxorubicin and paclitaxel. Results were calculated for each of these parameters if the level of activity was reached; however, if the effect was not achieved, the value was expressed as greater or less than the maximum or minimum concentration tested.
Based on Table 1, the synthesized compounds a-n showed potent to significant cancer cell growth inhibition with GI50 values ranging from <0.01 to >100 μM. Among tested compounds a exhibited potent anti-proliferative activity against IMR32 with GI50 value less than 0.01 μM, which was more active than standards Doxorubicin and Paclitaxel. Compound d showed anti-proliferative activity against IMR32 with GI50 value 0.1 μM, which was close to the standard Paclitaxel. Five compounds l, n, m, k and b exhibited promising anti-proliferative activity against IMR32 with GI50 values 0.21, 0.21, 0.69, 0.98 and 1.1μM. The Structure-Activity Relationship (SAR) revealed that. The presences of ester group/cyclic group on pyridine ring in combination with methyl group on C-2 position appear to be crucial for observed activity. Further studies are underway to optimize the lead molecule (Figure 1).
SRB Proliferative Assay Method
The cell lines, HeLa, MIAPACA, MDA MB 231 and IMR 32 (cervical, pancreatic, breast and neuroblastoma) which were used in this study were procured from American Type Culture Collection (ATCC), USA. The synthesized test compounds were evaluated for their in vitro anti-proliferative activity in these four different human cancer cell lines. A protocol of 48 h continuous drug exposure was used and an SRB cell proliferation assay was used to estimate cell viability or growth. All the cell lines were grown in Dulbecco’s modified Eagle’s medium (containing 10% FBS in a humidified atmosphere of 5% CO2 at 37 °C). Cells were trypsinized when sub-confluent from T25 flasks/60 mm dishes and seeded in 96-well plates in 100 μL aliquots at plating densities depending on the doubling time of individual cell lines. The microtiter plates were incubated at 37 °C, 5% CO2, 95% air, and 100% relative humidity for 24 h prior to addition of experimental drugs and were incubated for 48 h with different doses (0.01, 0.1, 1, 10, 100 μM) of prepared derivatives. After 48 h incubation at 37 °C, the cell monolayers were fixed by the addition of 10% (wt/vol) cold trichloroacetic acid and incubated at 4 °C for 1 h and were then stained with 0.057% SRB dissolved in 1% acetic acid for 30 min at room temperature. Unbound SRB was washed with 1% acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution for OD determination at 510 nm using a microplate reader (Enspire, Perkin Elmer, USA). Using the seven absorbance measurements [time zero, (Tz), control growth, (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth was calculated at each of the drug concentrations levels. Percentage growth inhibition was calculated as:
[(Ti-Tz)/(C-Tz)] x 100 for concentrations for which Ti>/=Tz
[(Ti-Tz)/Tz] x 100 for concentrations for which Ti>Tz
The dose response parameter, growth inhibition of 50% (GI50) was calculated from [(Ti-Tz)/(C-Tz)] x 100 = 50, which is the drugconcentration resulting in a 50% reduction in the net protein increase(as measured by SRB staining) in control cells during the drug incubation. Values were calculated for this parameter if the level of activity is reached; however, if the effect is not reached or is exceeded, the value for that parameter was expressed as greater or less than the maximum or minimum concentration tested.
Conclusion
Taken together, we have synthesized a series of benzo [6,7]cyclohepta [1,2-b] pyridine derivatives (a-n) in good yields and performed there in vitro anti-proliferative activity against four different human cancer cell lines, HeLa, MIAPACA, MDA-MB-231 and IMR32. Compound a exhibited potent anti-proliferative activity against IMR32 with GI50 value less than 0.01 μM, which was more active than standards Doxorubicin and Paclitaxel in addition to antiinflammatory activity.
References
- National Cancer Institute (1971).
- Hoffsomer RD, Taub D, Wendler NL (1962) The homoallylic rearrangement in the synthesis of amitriptyline and related systems. J Org Chem 27: 4134-4137.
- Schindler W, Hafliger F (1954) Uber derivate des iminodibenzyls. Helv. Chim. Acta 59, 472.
- Hoffmeister VF, Wutke WG, Kroneberg (1969) Zur Pharmakologie des thymolepticum noxiptilin. Synthesis of novel potentially biologically active dibenzosuberone derivatives. Arzneimittel Forsch 19: 846.
- Moffett RB (1964) Central nervous system depressants VII. Pyridyl coumarins. J Med Chem 7: 446-449.
- Sreenivasulu B, Sundaramurthy V, Subba Rao NV (1974) Search for physiologically active compounds. Proc Indian Acad Sci Sect A 79: 41-47.
- Krutosikova A, Sleziak R (1996) Synthesis of 2-Arylfuro[3,2-c]pyridines and their derivatives. Collect Czech Chem Commun 61: 1627-1636.
- Benckova M, Krutosikova A, Pullman J, Pronayova N (1999) Pyrrolo[2/,3/:4,5]furo[3,2-c]pyridine derivatives reactions in the pyridine and pyrrole ring. Chem Papers 53: 118.
- Ramesh D, Chandrashekhar C, Vaidya VP (2008) Synthesis of novel naptho[2,1-b]furo[3,2-b]pyridine derivatives as potential antimicrobial agents. Indian J Chem Section B 47: 753-758.
- Benckova M, Krutosikova A (1999) 5-Aminofuro[3,2-c]pyridinium Tosylates and Substituted Furo[3,2-c]pyridine N-Oxides: synthesis and reactions. Collect Czech Chem Commun 64: 539-547.
- Wang Y, Wang J, Lin Y, Si-Ma LF, Wang DH, et al. (2011) Synthesis and antihistamine evaluations of novel loratadine analogues. Bioorg Med Chem Lett 21: 4454-4456.
- Liu GZ, Xu HW, Chen GW, Wang P, Wang YN, et al. (2010) Stereoselective synthesis of desloratadine derivatives as antagonist of histamine. Bioorg Med Chem 18: 1626-1632.
- Soule BP, Simone NL, DeGraff WG, Choudhuri R, Cook JA, et al. (2010) Loratadine dysregulates cell cycle progression and enhances the effect of radiation in human tumor cell lines. Radiat Oncol 5: 8.
- Piwinski JJ, Wong JK, Green MJ, Ganguly AK, Billah MM, et al. Dual antagonists of platelet-activating factor and histamine. Identification of structural requirements for dual activity of N-acyl-4-(5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidines. J Med Chem 34: 457-461.
- Gelfand EW, Appajosyula S, Meeves S (2004) Anti-inflammatory activity of H1-receptor antagonists: review of recent experimental research. Curr Med Res Opin 20: 73-81.
- Agrawal DK (2004) Anti-inflammatory properties of desloratadine. Clin Exp Allergy 34: 1342-1348.
- Robak T, Szmigielska-Kaplon A, Pluta A, Grzybowska-Izydorczyk O, Wolska A, et al. (2011) Novel and emerging drugs for acute myeloid leukemia: pharmacology and therapeutic activity. Curr Med Chem 18: 638-666.
- Sajja Y, Vulupala HR, Bantu R, Nagarapu L, Vasamsetti SB (2016) Three-component, one-pot synthesis of benzo[6,7]cyclohepta [1,2-b]pyridine derivatives under catalyst free conditions and evaluation of their anti-inflammatory activity. Bioorg Med Chem Lett 26: 858-863.
- Nagarapu L, Bandi Y, Bantu R (2014) Synthesis of novel building blocks of benzosuberone bearing coumarin moieties and their evaluation as potential anticancer agents. Eur J Med Chem 79: 260.
- Nagarapu L, Bandi Y, Bantu R (2014) Studies on the synthetic and structural aspects of benzosuberones bearing 2, 4 thiazolidenone moiety as potential anti-cancer agents. Eur J Med Chem 71: 91.
- Nagarapu L, Vulupala HR, Bantu R, Sajja Y, Nanubolu JB (2014) Efficient synthesis and resolution of novel 2-(hydroxymethyl)-7,8-dihydro-1H-indeno[5,4-b] furan-6(2H)-one by lipase pseudomonas cepacia. Tetrahedron Asymmetry 25: 578-582.
- Nagarapu L, Vanaparthi S, Bantu R, Ganesh Kumar C (2013) Synthesis of novel benzo[4,5]thiazolo[1,2-a]pyrimidine-3-carboxylatederivatives and biological evaluation as potential anticancer agents. Eur J Med Chem 69: 817.
- Nagarapu L, Shuklachary K, Bantu R (2012) Total synthesis of sapinofuranone A from D-ribose. Tetrahedron 68: 5829-5832.
- Nagarapu L, Mallepalli R, Nikil Kumar U, Paparaju P, Bantu R, et al. (2012) Synthesis of α1-oxindole- α-hydroxyphosphonates under catalyst-free conditions using polyethylene glycol (PEG-400) as an efficient and recyclable reaction medium. Tetrahedron Lett 53: 1699-1700.
- Nagarapu L, Raghu M, Lingappa Y, Bantu R (2011) Polyethylene glycol (PEG-400) as an efficient and recyclable reaction medium for one-pot synthesis of polysubstituted pyrroles under catalyst-free conditions. Tetrahedron Lett 52: 3401-3404.
- Mallepalli R, Yeramanchi L, Bantu R, Nagarpu L (2011) Polyethylene glycol (PEG-400) as an efficient and recyclable reaction medium for the one-pot synthesis of N-substituted azepines under catalyst-free conditions. Synlett 2730-2732.
- Nagarapu L, Paparaju V, Satyander A, Bantu R (2011) Total synthesis of (+) -varitriol via a symmetrical furanose diol as the key intermediate. Tetrahedron Lett 52: 7075.