Natural and Synthetic Molecular Medicine in Cancer Research: A Review
Hardesh K Maurya*
Medicinal Chemistry Department, CSIR-Central Institute of Aromatic and Medicinal Plants, Picnic Spot road, Lucknow-226015, India
Corresponding author: Hardesh K Maurya, Medicinal Chemistry Department, CSIR-Central Institute of Aromatic andMedicinal Plants, Picnic Spot road, Lucknow-226015, India; Telephone No.: +919451407702, E-Mail: hardesh11@yahoo
Citation: Maurya HK. Natural and Synthetic Molecular Medicine in Cancer Research: A Review. J Cancer Res Molecul Med. 2014;1(1): 101.
Copyright © Hardesh K Maurya 2014. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cancer Research and Molecular Medicine | Volume: 1, Issue: 1
Submission: Submission: 21/10/2014; Accepted: 08/12/2014; Published: 10/12/2014
Abstract
This review presents the stimulating area of natural and synthetic anticancer drugs which are presently used for the cure of various type of cancer. In this review, twenty one naturally, semisynthetic naturally occurring and 109 synthetic anticancer drugs are reported. The emphasis of this review has described related structure, mechanism of action and major disadvantage of these drugs with the aims of providing an overview of anticancer drugs. This review will be provide current status of cancer drug and helpful to make strategy in the search of novel anticancer molecular medicine in future.
Keywords: Anticancer drugs; Chemotherapy; Cancer; Carcinoma; Tumor; Cancer medicine
Introduction
Cancer is a disease with abnormal cell growth of any part ofour body. It is also called malignancy. Cancer’s cells are divideduncontrollably and forms lumps. These lumps are called tumor and harms our body.
On the bases of part of our body more than hundred type carcinoma cells have been reported [1,2]. The most common cancer type are bladder cancer, lung cancer, breast cancer, melanoma (cancer of pigment making cells), colon and rectal cancer, non Hodgkin lymphoma (cancer of white blood cells), endometrial cancer (cancer of uterus), pancreatic cancer, kidney cancer, prostrate cancer, leukemia (cancer of blood forming tissue e.g. bone marrow), thyroid cancer etc [3].
For the treatment of these cancers, various methods were applied such as surgery (a method to remove tumors from body), radiation therapy (a method to destroy or damage carcinoma cell though applies the high wave radiation), targeted therapy (other substances or useing drugs to more accurately discover and attack carcinoma cells), immunotherapy (increase immune system to fight cancer cells), hyperthermia (using heat to treat cancer), Stem cell transplant (stem cells were transplant to treat cancer cells), photodynamic therapy (photosensitizing agents are a special drugs and used along with light to kill cancer cells), lasers in cancer treatment, blood product donation and transfusion, and most important tools chemotherapy (use of medicine to treat cancer) [2]. Cancer therapies are not secured to cure cancer completely. Some cases, it secure to cure cancer completely but it is much costly and maximum population cannot efforts such treatment. Thus maximum patients were died without proper treatment in world. In 2014, 1.66 million new case and 0.556 million deaths have been reported in USA [4], while 14.1 million new case and 8.2 million deaths were estimated in 2012. Cancer is second most common disease (0.806 million) [5] in world as well as in India [6]. These results showing that various therapy are not sufficient to cure this deadly disease.
The future of chemotherapy is only a way to control this disease. More than hundreds molecular medicines are available in market. Current review is a compilation of structures, type of cancer therapy, mechanism of action and major disadvantage of these drugs with the aims of providing an overview of anticancer drugs for further modification in the search of potent anticancer molecular medicine.
Molecular Medicines
However, various molecular medicines are containing nitrogen atoms; therefore, I have classified these medicines on the basis of nitrogen atoms present in drugs.
Natural and semi synthetic medicines
a) One nitrogen containing natural and semi synthetic medicines (Tables 1-4)
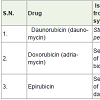
Daunorubicin (daunomycin) is an anthracycline antibiotic which was isolated from Streptomyces peucetius [7-9]. It is used to treat acute lymphocytic and myelocytic leukemia since 1960 with the side effects such as hair loss, low white blood cells, vomiting, nausea, decrease platelet counts etc..
Doxorubicin (adriamycin) [10,11]is a semi synthetic medicine of daunorubicin. It is used to treat various type of cancer such as Hodgkin’s lymphoma (a white blood cells’ cancer), acute leukemia, multiple myeloma, Ewing sarcoma, Kaposi sarcoma, breast, ovary, lung, endometrium, adrenal cortex and other cancers with side effect cardiomyopathy (heart muscles disease) with heart failure, hair loss, low white blood cells, vomiting, nausea, decrease platelet counts etc.
Epirubicin [12] (an anthracycline analog) was synthesized from 13-dihydrodaunorubicine (13-daunorubicinol). It is used to treatment of breast cancer with the combination of other drugs and it has low side effects as comparison with doxorubicin.
Daunorubicin, doxorubicin and epirubicin acts as DNA cleavage of carcinoma cells by topoisomerase II inhibitor.
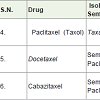
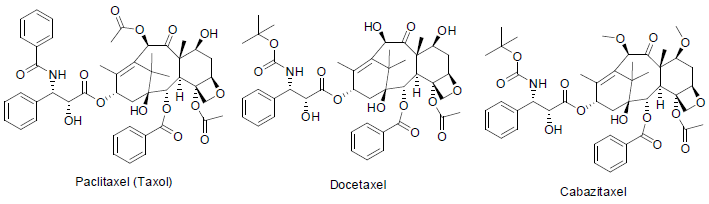
Paclitaxel (Taxol) [13] was isolated from the inner bark of Taxus brevifolia (Pacific yew tree) in 1967. It is used to treatment of long, breast, ovarian, neck, head, Kaposi (a tumor caused by herpes virus) carcinoma with the side effects such as hair loss, low white and red blood cells, vomiting, nausea, decrease platelet counts, diarrhea, numbness, pain in joint etc.
Docetaxel is a semi-synthetic medicine, synthesized from paclitaxel and used to treatment of breast [14], lung, prostate, stomach, head and neck cancers with the side effects such as fever, low white blood cell count, hair loss, diarrhea, nausea, tiredness, anemia, menstrual cycle abnormality in women, skin rash etc.
Cabazitaxel is also a semi-synthetic derivative of taxol and approved to cure prostate cancer [15] since 2010 with similar side effects as indocetaxel.
Paclitaxel, Docetaxel and Cabazitaxel are grouped as taxane and act as microtubule inhibitors (stop the formation of microtubule during the cancer’s cell division).
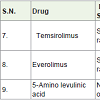
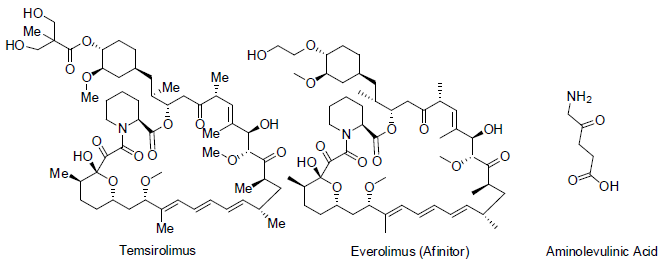
Temsirolimus [16] is a semi synthetic medicine of sirolimus (also called rapamycin). Sirolimus was isolated from the soil bacteria Streptomyces hygroscopicus. Temsirolimus was approved to cure of renal cell carcinoma (kidney cancer) in 2007 with the side effects of skin rash, tiredness, mouth sores, nausea, swelling in hand, loss of appetite, anaemia, low white blood cell count, high blood sugar, high blood cholesterol etc.
Everolimus [17] is also a semi synthetic medicine of sirolimus and approved to cure renal cell carcinoma in 2009 with similar side effects as temsirolimus.
Temsirolimus and everolimus acts as mTOR inhibitors (mechanistic target of rapamycin). In which these drugs are interface the synthesis of proteins that control the proliferation, growth and survival of cancer cells.
5-Aminolevulinic acid [18] is a natural product intermediate in the formation of porphyrin and also found in insects, fungi, some bacteria, protozoans etc. Due to fluorescent characteristic, it is used detection of cancer and also as photodynamic therapy for various type cancers.
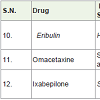
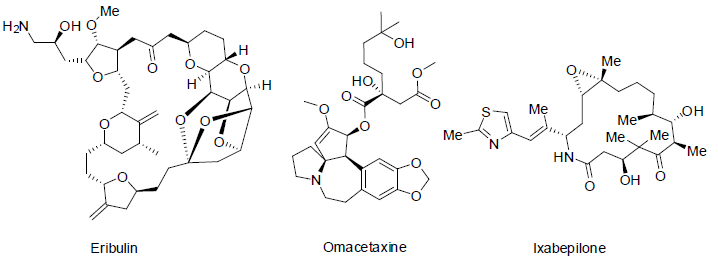
Eribulin [19] is a synthetic analogue of natural product halichondrin B, which was isolated from the marine sponge Halichondria okadai. Eribulin was approved to treatment of metastatic breast cancer (4th state breast cancer) in 2010 with the side effects low white blood and red cell count, numbness, hair loss, tiredness, weakness, nausea, joint and muscle pain, weight loss, loss of appetite, constipation, fever etc. Eribulin acts as microtubule inhibitors of cancer cells.
Omacetaxine [20,21] is a semi synthetic derivative of the alkaloid cephalotaxine which was isolated from Cephalotaxus harringtonia. It is approved to treatment of chronic myelogenous (chronic granulocytic), leukemia (a white blood cell cancer) in 2012 with following side effects anemia, low blood platelet count, low white blood cell count, diarrhea, nausea, pain, tiredness, fever, weakness etc and it works as tyrosine kinase inhibitors.
b) Two nitrogen containing natural and semi synthetic medicines (Table 4)
Ixabepilone (azaepothilone B) is isolated by Sorangium cellulosum (a soil-dwelling gram-negative bacterium). Ixabepilone [22] had shown very high potential at very low concentration as microtubule inhibitors and approved to treatment of metastatic breast cancer in 2007 with side effects hair loss, diarrhea, mouth sores, vomiting, nausea, join and muscle pain, tiredness, numbness, low white blood cell count etc.
c) Four nitrogen containing natural and semi synthetic medicines (Table 5,Table 6)
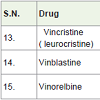
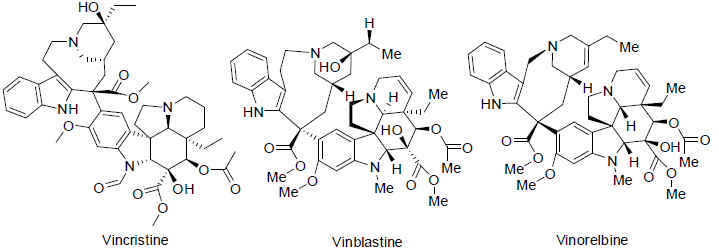
Vincristine ( leurocristine) was isolated from the Catharanthus roseus (a vinca alkaloid) [23]. Vincristine is used to treatment of leukemias, hodgkin’s lymphomas and childhood cancers with side effects constipation, tiredness, hair loss, pain etc. Vinblastine was also obtained from Catharanthus roseus (vinca rosea, a madagascar periwinkle) and used to treatment of Hodking’s lymphomas, breast, lung, testicular (a man reproductive system) head and neck cancers with side effects such as mouth sores, tiredness, low blood platelet count, low white blood cell count etc. Vinorelbine is a semi-synthetic vinca alkaloid and approved for the treatment of non small cell lung and breast cancer in 1989 with side effects constipation, tiredness, weakness, vomiting, nausea, anemia, low white blood cell count etc. Vincristine, vinblastine and vinorelbine are worked as mitotic inhibitors (as antimicrotubule) and inhibit the formation mitosis of carcinoma cells.
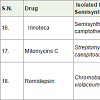
Irinoteca is a semi-synthetic medicine of the natural alkaloid camptothecin [24] and approved to treatment of colon and rectal cancer which worked as topoisomerase I inhibitors with the side effects such as diarrhea, hair loss, appetite loss, nausea, vomiting, abdominal pain, weakness, low white blood cell count etc.
Mitomycins C [25] are a member of mitomycins natural products family, isolated from Streptomyces caespitosus or Streptomyces lavendulae and Mitomycins C is an antibiotics and worked as alkylating agents for the treatment of stomach, colon, rectal, pancreatic as well as breast, cervical, lung, head and neck cancers with the side effects such as hair loss, tiredness, low white blood cell count, low platelet count etc.
Romidepsin [26] (a depsipeptide) is a natural product obtained from the bacteria Chromobacterium violaceum. It is approved for the treatment for cutaneous T-cell lymphoma (a skin cancer), peripheral T-cell lymphoma in 2005 as histone deacetylase inhibitor with various side effects such as constipation, diarrhea, rashes on skin, other infection, increase blood sugar, anemia, low platelet count, low white blood cell count, appetite loss, tiredness, poor food taste, change in blood mineral level etc.

d) Five and more nitrogen containing natural and semi synthetic medicines (Table 7)
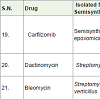
Carfilzomib (a tetrapeptide epoxyketones) is semisynthetic drug of a natural product epoxomicin. Carfilzomib [27] was approved for the treatment of multiple myeloma (cancer of plasma cell) in 2012 and acts as proteasome inhibitors (block the action of proteasomes) with the side effects such as fatigue, anemia, low platelet count, nausea, vomiting, shortness in breathing, fever, diarrhea, cold, cough, headache, diarrhea, swelling, back pain, constipation, low white blood cell count, increase of blood creatinine level etc.
Dactinomycin [28] (actinomycin D) is a polypeptide antibiotics which was isolated from soil bacteria of the genus Streptomyces. Dactinomycin was approved to treatment of Wilms tumors (a type of children’s kidney tumors), Ewings tumors of bone or soft tissues, testicular cancer, sarcomas (in cartilage, fat, muscle or bone) in 1964 and acts as transcription inhibitors (stop cancer cells growing) with the side effects such as radiation effects, hair loss, diarrhea, rash, skin darking, mouth sores, appetite loss, low platelet count, vomiting, nausea, low white blood cell count.
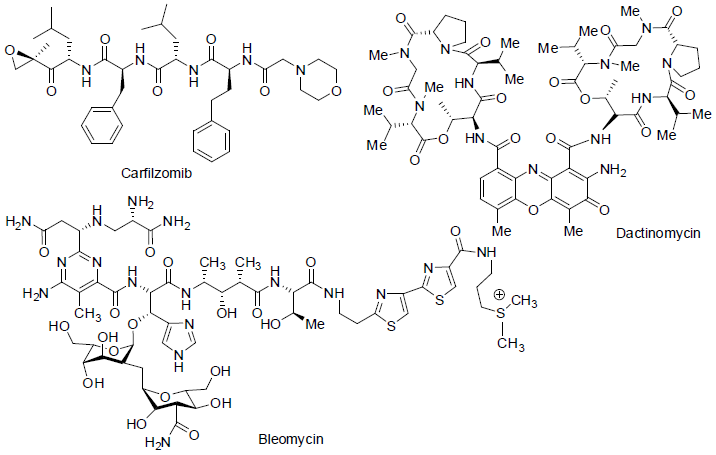
Bleomycin (a glycopeptide) is an antibiotic which was isolated from the bacterium Streptomyces verticillus. Bleomycin [29,30] is used to treatment of Hodgkin’s sarcoma, lymphoma, squamous cell cancer of the head, neck, cervix, testicular cancer etc and acting as DNA breaking of cancer cells with side effects such as fever, chill, nausea, vomiting, appetite loss, hair loss, mouth sores, darkened skin etc.
Synthetic molecular medicines
a) One nitrogen containing synthetic medicines (Table 8,Table 9)
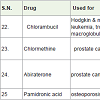
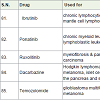
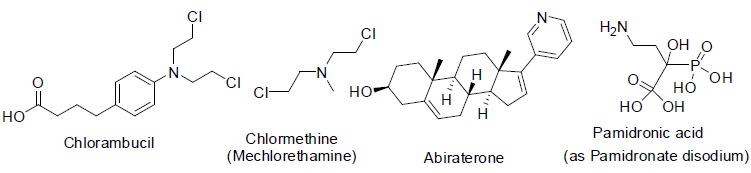
Chlorambucil [31] is a nitrogen mustard alkylating agents (act as binding DNA) approved before 1984 for the treatment of Hodgkin and non-Hodgkin lymphoma, chronic lymphocytic leukemia, trophoblastic neoplasms, waldenström macroglobulinemia (a white blood cell carcinoma), polycythemia vera (formation of more red blood cell) and ovarian carcinoma with side effects low white blood cells count, low platelet count, menstrual period stop, sperm reduction, hair loss etc.
Chlormethine (mechlorethamine, mustine) is also nitrogen mustard alkylating agents and used to treatment of prostate cancer since 1946[32] with side effects such as low white blood cell count, low platelet count, anemia, nausea, vomiting, tasteless, low appetite, hair loss, longterm infertility etc.
Abiraterone was approved to treatment of advanced prostate cancer in 2011 however [33] and works as 17 α-hydroxylase/C17, 20 lyase (CYP17A1) inhibitor with side effects such as joint pain, swelling, tiredness, muscle aches, low blood levels of potassium or phosphate, high cholesterol, anemia, low white blood cell count, abnormal blood test results, high blood sugar etc.
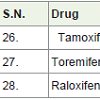
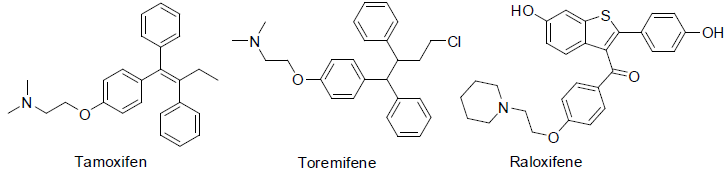
Tamoxifen is an essential medicine with very less side effects as comparison with other anticancer drugs and it is used to treatment of breast cancer [34]. It is a special type hormone prodrug, used as estrogen receptor and known as selective estrogen receptor modifier (SERM).
Toremifene [35] was approved for the treatment of breast cancer in 1997 and also used for the treatment of prostate cancer and acts as selective estrogen receptor modifier (SERM) similar to tamoxifen.
Raloxifene [36] is also a selective estrogen receptor modifier (SERM) and approved to treatment of breast cancer in 2007. Tamoxifen, toremifen and raloxifene were showing common side effects such as hot flashes, tiredness and leg cramps.
b) Two nitrogen containing synthetic medicines (Tables 10-12)
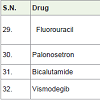
Fluorouracil (5-FU) [37] is an important essential drug of varies cancer treatment such as breast, anal, stomach, colorectal, pancreatic, skin and oesophageal. It acts as thymidylate synthase inhibitors with side effects such as nausea, headache, vomiting, hair loss, photosensitivity low white blood cell count, low platelet count, sores in mouth, brittle nails, dry skin etc.
Palonosetron [38] is an anti-nausea 5HT3 antagonist medicine which is used to prevent vomiting and nausea after chemotherapy.
Bicalutamide [39] is a hormone antagonist (block androgen male hormone) and approved to treatment of prostate cancer in 1995 with side effects swelling, hot flashes, pain in breast etc.
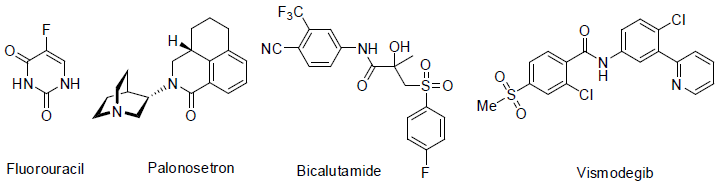
Vismodegib [40] was approved to treatment of basal cell carcinoma (a skin cancer) in 2012. It acts as hedgehog signaling pathway antagonist with side effects hair loss, weight loss, nausea, muscle spam, tiredness, change in taste, diarrhea etc.
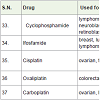
Cyclophosphamide [41] is nitrogen alkylating agent which used for the treatment of lymphoma, multiple myeloma, leukemia, neuroblastoma, mycosis fungoides, retinoblastoma etc with side effects hair loss, diarrhea, nausea, low white blood cell count, mouth sores, appetite loss, bleeding from bladder, vomiting etc.
Ifosfamide [42] is a nitrogen mustard alkylating agents and used for the treatment of breast cancer, lung cancer, cervical cancer, testicular cancer, ovarian cancer, lymphoma, bone cancer with side effects hair loss, diarrhea, nausea, low white blood cell count, mouth sores, appetite loss, bleeding from bladder, vomiting etc.
Cisplatin [43] (cis-diamminedichloroplatinum(II), cisplatinum) is a platinum containing anticancer drug which acts as cross linking of DNA (alkylating agents) of carcinoma cells and approved to treatment of ovarian, testicular, bladder, lung cancer with side effect kidney damage, decreased potassium, magnesium and calcium level in blood, vomiting, nausea, low white blood count, low platelet count, anemia, change in taste, numbness, swelling etc.
Oxaliplatin [44] is also a platinum containing drug which was approved to cure colorectal cancer in 2012 and acts cross linking of DNA (alkylating agents) of carcinoma cells with side effects such as vomiting, numbness, nauea, abdominal pain, mouth sores, tiredness, diarrhea etc.
Carboplatin [45] (a platinum containing drug) was approved to treatment of ovarian, lung, head, neck etc carcinoma in 1989. It acts as cross linking of DNA (alkylating agents) of carcinoma cells with side effects such as low white blood cell count, low platelet count, anemia, brittle hair, abnormal kidney function etc.
Thalidomide [46] was approved to treatment of multiple myeloma (bone marrow cancer) in 2006. It is an immunomodulating agents with various side effects tiredness, rash, low white blood cell counts sleepiness, dizzy feeling etc.
Vorinostat [47] was approved to treatment of cutaneous T-cell lymphoma (a skin cancer) in 2006 and acts as histone deacetylase inhibitors with side effect tiredness, diarrhea, vomiting, nausea, appetite loss, weight loss, low blood platelet count, high blood sugar, muscle arches, food taste change.

c) Three nitrogen containing synthetic medicines (Tables 13-15)
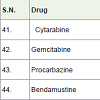
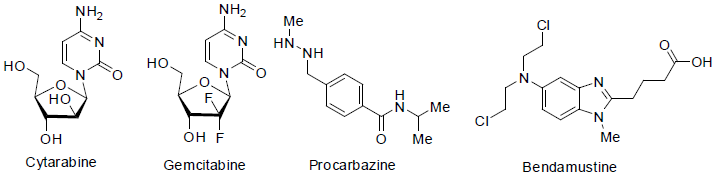
Cytarabine is a most essential drug which is used to cure acute myeloid leukemia (a white blood cell cancer) and non-hodgkin lymphoma. Itacts as DNA synthesis inhibitors of carcinoma cell with a side effects such as nausea, vomiting, low blood cell count, low platelet count, stomach pain, tiredness, mouth sore etc [48].
Gemcitabine is used to treatment of various type cancers such as pancreatic, lung [49], ovarian, breast cancers. It also acts as DNA synthesis inhibitors of carcinoma cells with side effects such as fever, low white blood cell count, low platelet count, anemia, nausea, vomiting, tiredness, rash on skin, appetite loss etc.
Procarbazine is an alkylating agent and approved to treatment of Hodgkin’s lymphoma and glioblastoma (brain cancer) in 1969 with side effects low white blood cell count, low platelet count, depression, nausea, vomiting, felling nervousness, appetite loss, sleeping problems etc. It is used with combination of other anticancer medicines [50].
Bendamustine [51] was approved to treatment of chronic lymphocytic leukemia and non Hodgkin lymphoma in 2008. It is a nitrogen mustard and acts as alkylating agents with side effects low white blood cell count, low platelet count, anemia, fever, nausea, vomiting, diarrhea, appetite loss, tiredness etc.
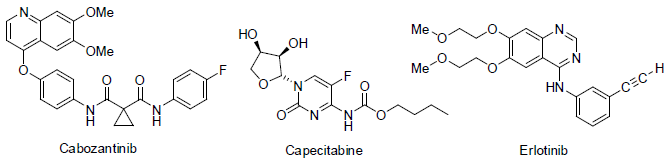
Cabozantinib [52] was recently approved (2012) for the treatment of modularly thyroid cancer. It acts as targeted tyrosine kinase inhibitors with side effects weight loss, diarrhea, mouth sores, nausea, appetite loss, weakness, abdomen pain, vomiting, high blood pressure, change in voice etc.
Capecitabine [53] was used to treatment of numerous type cancers such as breast cancer, colon, rectal etc. It acts as anti metabolized with side effects such as fever hair loss, diarrhea, headache, nausea, appetite loss, weakness, abdomen pain, vomiting, eye irritations.
Erlotinib [54] was approved to treatment of pancreatic and non small lung cancer in 2004. It acts as epidermal growth factor receptor (EGFR, a tyrosine kinase inhibitor) with side effects such as skin rash, nausea, diarrhea, tiredness, appetite loss.
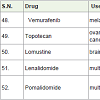
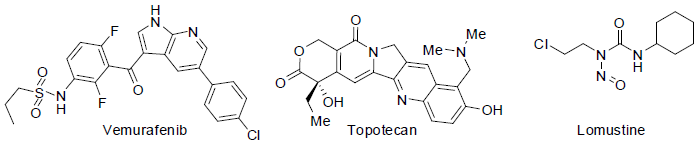
Vemurafenib [55] was approved to treatment of melanoma (a skin cancer) in 2011. It acts as B-raf enzymes inhibitors with side effects such as hair loss, pain in joint, rash, warts, tiredness, headache, sunlight sensitivity, diarrhea, nausea etc.
Topotecan [56] was approved for the treatment of ovarian, cervical and lung cancers in 1996. It acts as topoisomerase I inhibitors with side effects such as nausea, vomiting, hair loss, low white blood cell count, low blood platelet count, anemia etc.
Lomustine [57] was used to treatment of brain cancer due to high lipid solubility. It acts as alkylating agents with side effect such as vomiting, nausea, fatal disorder during pregnancy, low white blood cell count, low blood platelet count etc.
Lenalidomide [58] was approved to treatment of multiple myeloma in 2005 with side effects such as tiredness, rash, itching, diarrhea, low white blood cell count, low blood platelet count etc.
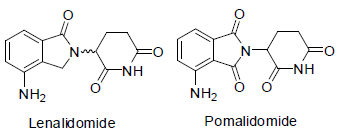
Pomalidomide [59] was approved to treatment of multiple myloma in 2013. Possible, it acts as angiogenesis inhibitors with side effects such as tiredness, back pain, anemia, diarrhea, nausea, chest pain, appetite loss, pneumonia, breathing problem, rash, low white blood cell count, low blood platelet count etc.
d) Four nitrogen containing synthetic medicines (Tables 16-19)
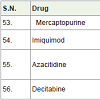
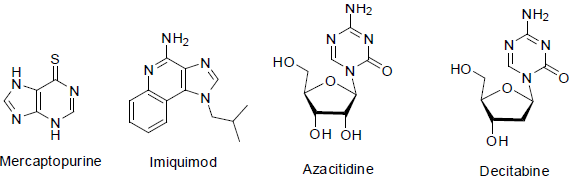
Mercaptopurine (azathioprine) [60] is used for the treatment of leukemia (a cancer of bone marrow). It acts as anti-metabolite with side effects such as low white blood cell count, low blood platelet count and anemia etc.
Imiquimod [61] was approved in 1997 and it is used as cream for the treatment of skin cancers with the change of immune response and side effects such as skin redness, sunburns, drying skin etc.
Azacitidine [62] was first approved in 2004. It is used for the treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia which act as anti-metabolite with side effects such as anemia, low platelet count, vomiting, fever, nausea and tiredness etc.
Decitabine [63] was approved in 2006 for the treatment of myelodysplastic syndromes and acts as hypomethylating agents with side effects such as anemia, low platelet count, low white blood cell count, vomiting, diarrhea, fever, nausea, pain in legs and tiredness etc.
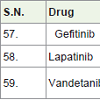
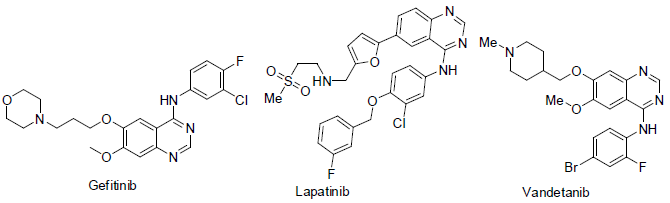
Gefitinib [64] was approved in 2003. It used to treatment of breast and lung cancer. It acts as epidermal growth factor receptor (EGFR) for a tyrosine kinase protein with side effects such as skin rash and diarrhea etc.
Lapatinib [65] was approved to treatment of breast cancer. It acts as tyrosine kinase inhibitor with side effects diarrhea, rashes, swelling, numbness and pain in hand and foot.
Vandetanib [66] was approved in 2011 for the treatment of thyroid cancers. It acts as kinase inhibitor with side effects diarrhea, nausea, tiredness, appetite loss, abdomen pain, headache, high blood pressures, rashes etc.
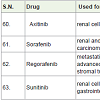
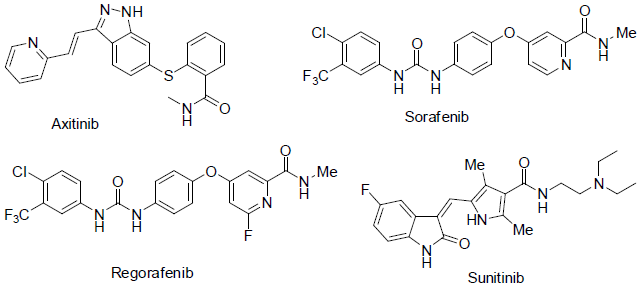
Axitinib [67] was approved in 2012 for the treatment of renal cell carcinoma. It acts as vascular epidermal growth factor receptor (VEGFR) for a tyrosine kinase protein with side effects such as skin rashes, diarrhea, nausea, low blood cell counts, tiredness, appetite loss, weight loss, swelling, numbness and pain in hand and high blood pressures etc.
Sorafenib [68] was approved in 2005 for the treatment of renal and hepatocelluar carcinoma. It also acts as oral receptor tyrosine kinase inhibitor with side effects tiredness, diarrhea, itching, rashes, swellings in hand and feet etc.
Regorafenib [69] was approved in 2012 for the treatment of metastatic colorectal and advanced gastrointestinal stromal tumors. It acts as oral receptor tyrosine kinase inhibitor with side effects fever, appetite loss, tiredness, weakness, diarrhea, infection, weight loss, moth’s sores, rashes, high blood pressure, change in voice, bleeding etc.
Sunitinib [70] was approved in 2006 for the treatment of renal cell carcinoma and gastrointestinal carcinoma. It acts as tyrosine kinase receptor with side effects such as low white blood cell count, nausea, vomiting, diarrhea, mouth sore, appetite loss, taste of food change, tiredness, weakness, and change in hair and skin color, high blood pressure, hair loss etc.
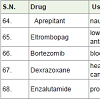
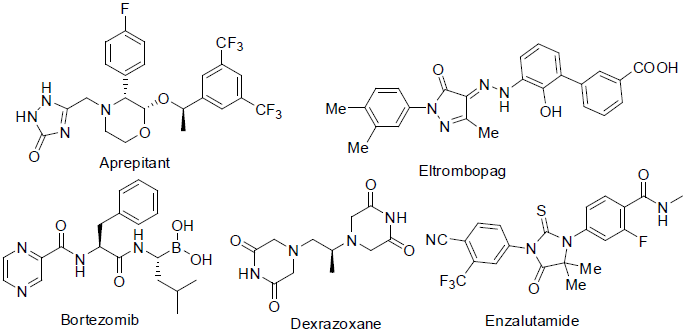
Aprepitant [71] was approved in 2003 for the treatment of nausea caused by various anticancer drugs with side effects such as diarrhea, itching, appetite loss etc.
Eltrombopag [72] was approved in 2008 for the treatment of low plate count (thrombocytopenia) caused by various anticancer drugs.
Bortezomib [73] was approved in 2003 for the treatment of blood and bone marrow (multiple myloma and mantle cell lymphoma) cancer.
It acts as proteasome inhibitor with side effects such as weakness, nausea, vomiting, tiredness, diarrhea, constipation etc.
Dexrazoxane [74] was approved in 1995 to protect heart damage caused by anti-cancer drug doxorubicin with side effect low white blood cell count and low blood platelet counts.
Enzalutamide [75] was approved in 2012 for the treatment of prostate cancer. It acts an androgen receptor antagonist (blocking the male hormones androgens) with side effects such as tiredness, weakness, diarrhoea, joint pain, hot flashes, swelling in head etc.
e) Five nitrogen containing synthetic medicines (Tables 20-23)
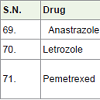
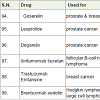
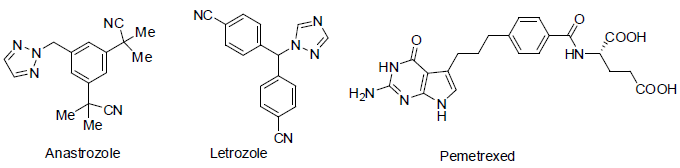
Anastrazole [76] was approved in 1995 for the treatment of breast cancer. It acts as aromatase inhibitors (block aromatase which is necessary for the formation of estrogen) with side effects such as pain in joint and bones, hot flashes etc.
Letrozole [77] was approved in 1997 for the treatment of breast cancer. It also acts as aromatase inhibitors with side effects such as weakness, pain in joint and bones, hot flashes etc.
Pemetrexed [78] was approved in 2004 and used for the treatment of malignant pleural mesothelioma (the lining of the chest cavity around the lungs cancer) and non small cell lung cancer. It acts as anti-metabolites with side effects such as breathing difficulty, tiredness, rashes, appetite loss, mouth sores, constipation, nausea, vomiting, low platelet count, low white blood cell count, anemia etc.
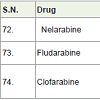
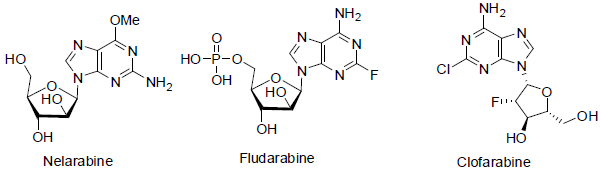
Nelarabine [79] was approved in 2005 and used for the treatment of T-cell acute lymphoblastic leukemia (a white blood cell cancer). It also acts as anti-metabolites (block the formation of DNA and RNA of carcinoma cells) with side effects such as headache, tiredness, low platelet count, low white blood cell count, anemia etc.
Fludarabine [80] was approved in 1991 and used for the treatment of cancers of blood cells lymphomas and leukemias (hematological malignancies). It also acts as anti-metabolites with side effects such as tiredness, low platelet count, low white blood cell count, anemia, vomiting, nausea, infections, fever etc.
Clofarabine [81] was approved in 2004 and used for the treatment of acute lymphoblastic leukemia, acute myeloid leukaemia and juvenile myelomonocytic leukaemia. It acts a anti-metabolites with side effects such as tiredness, low platelet count, low white blood cell count, anemia, vomiting, nausea, infections, fever etc.
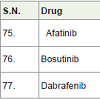
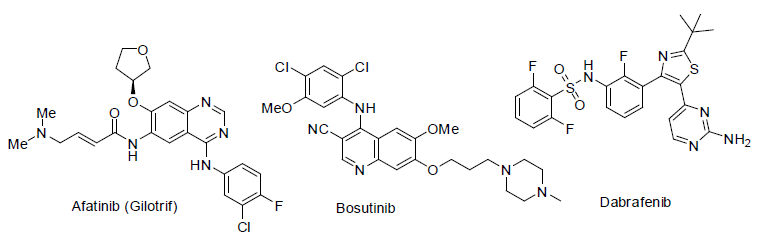
Afatinib (Gilotrif) [82] was approved in 2013 and used for the treatment of non small cell lung carcinoma. It acts as tyrosine kinases inhibitors with side effects scratchy and dry skin, appetite loss, infections in around nails skin, sores in mouth, rashes in skin, diarrhea etc.
Bosutinib [83] was approved in 2012 and used for the treatment of chronic myelogenous leukemia (a white blood cell carcinoma). It also acts as tyrosine kinases inhibitors with side effects headache, cough, anemia, low white blood cell count, tiredness, fever, vomiting, nausea, diarrhea, rashes in skin, abdomen pain etc.
Dabrafenib [84] was approved in 2013 and used for the treatment of melanoma caused by changing in BRAF gene (a gene responsible for the formation of B-Raf protein). It acts as kinase inhibitors (inhibit associated enzyme B-Ref) with side effects high blood sugar, fever, pain in joint, warts, hair loss, headache, skin thickness, etc.
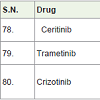
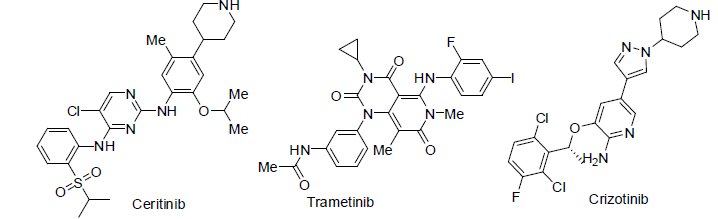
Ceritinib [85] was approved in 2014 and used for the treatment of non small cell lung carcinoma. It acts as anaplastic lymphoma kinase (ALK) inhibitor with side effects anemia, low white blood cell count, constipation, tiredness, appetite loss, abdomen pain, diarrhea, nausea, vomiting etc.
Trametinib [86] was approved in 2013 and used for the treatment of melanoma caused by changing in BRAF gene. It acts as kinase inhibitors with side effects anemia, low blood albumin level, rashes in skin, diarrhea, swelling etc.
Crizotinib [87] was approved in 2011 and used for the treatment of non small lung cancer. It acts as anaplastic lymphoma kinase (ALK) inhibitor with side effects rashes in diarrhea, nausea, vomiting, vision disorder, constipation, appetite loss, breathing problem, cold, swelling, nerves disorders etc.
f) Six nitrogen containing synthetic medicines (Table 24)
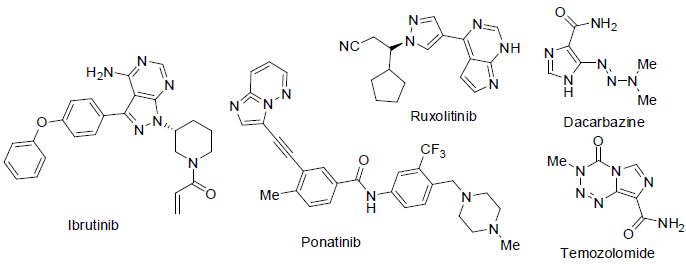
Ibrutinib [88] was approved in 2013 and used for the treatment of chronic lymphocytic leukemia and mantle cell lymphoma (a non-Hodgkin’s lymphomas). It acts as tyrosine kinase inhibitor with side effects nausea, vomiting, diarrhea, tiredness, pain in bone and muscles, cold, swelling, breath disorder, rashes in skin, constipation, abdominal pain, appetite loss, anemia, low platelet count etc.
Ponatinib [89] was approved in 2012 and used for the treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). It acts tyrosine kinase inhibitor with side effects high blood pressure, heart attack disorder, dry skin, rashes in skin, abdomen pain, tiredness, weakness, headache, constipation, pain in joint and muscle, fever, nausea, low platelet count, low white blood cell count etc.
Ruxolitinib [90] was approved in 2011 and used for the treatment of myelofibrosis (a bone marrow carcinoma) and pancreatic carcinoma. It acts as Janus kinase inhibitor (JKI) with side effects low blood platelet counts, fatigue, anemia, diarrhea, breath disorder, headache, nausea, dizziness etc.
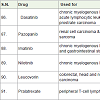
Dacarbazine [91] is an alkylating agents used for the treatment of Hodgkin lymphoma, malignant melanoma, islet cell carcinoma of the pancreas and sarcoma with side effects low white blood cell count, low platelet count, vomiting, nausea, hair loss, appetite loss, tiredness, headache, fever etc.
Temozolomide [92] was approved in 1999 and used for the treatment of glioblastoma multiforme (brain tumor) and melanoma (skin cancer). It acts as alkylating agents with side effects low white blood cell count, low platelet count, weakness, vomiting, nausea, hair loss, appetite loss, constipation, tiredness, headache, pain, itching etc.
g) Seven nitrogen containing synthetic medicines (Table 25)
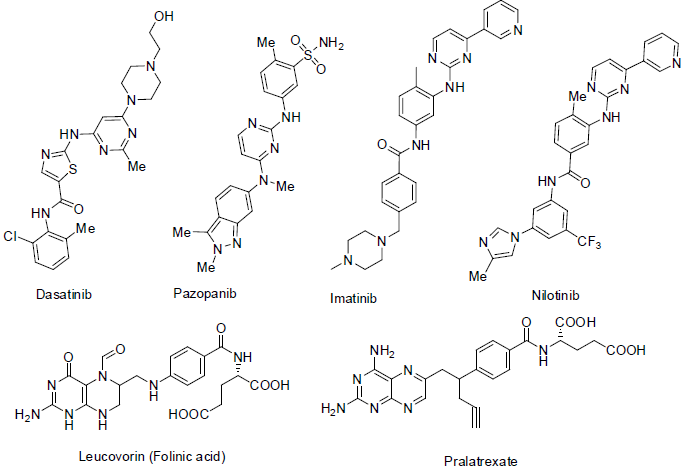
Dasatinib [93] was approved in 2006 and used for the treatment of chronic myelogenous leukemia, acute lymphocytic leukemia and prostrate carcinoma. It acts a tyrosine kinase inhibitor with side effects low white blood cell count, low blood platelet count, breath disorder, rashes in skin, pain in bone and muscle, headache, fever, tiredness, nausea, diarrhea, swelling etc.
Pazopanib [94] was approved in 2009 and used for the treatment of renal cell carcinoma (kidney cancer) and soft tissue sarcoma (a connective tissue carcinoma). It acts as tyrosine kinase inhibitor with side effects slow heart beat, weight loss, pain in joint and muscles, abdomen pain, change in taste, appetite loss, tiredness, high blood pressure, headache, vomiting, diarrhea, nausea etc.
Imatinib [95] was approved in 2001 and used for the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors. It acts as tyrosine kinase inhibitor with similar side effects of pazopanib.
Nilotinib [96] was approved in 2007 and used for the treatment of chronic myelogenous leukemia. It acts as tyrosine kinase inhibitor with side effects low white blood cell count, low blood platelet count, tiredness, headache, fever, diarrhea, constipation, nausea, vomiting, rashes in skin, itching etc.
Leucovorin [97] is used as combinatorial chemotherapy drug with 5-fluorouracil for the better treatment of colorectal, head and neck carcinoma.
Pralatrexate [98] was approved in 2009 and used for the treatment of peripheral T-cell lymphoma (a non-Hodgkin lymphoma). It acts as anti-metabolites with side effects mouth sore, nausea, vomiting, diarrhea, anemia, low white blood cell count, low platelet count, tiredness, constipation, fever, cough, swelling, nose bleeding etc.
h) Eight nitrogen containing synthetic medicines (Table 26)
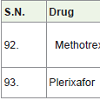
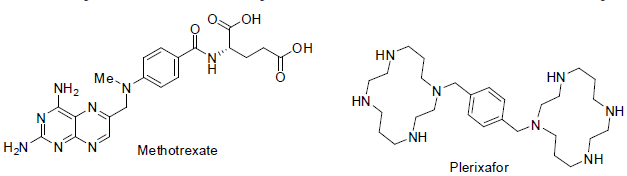
Methotrexate [99] is used for the treatment of head and neck, breast, lymphoma, leukemia, osteosarcoma, lung, bladder and trophoblastic neoplasms carcinoma. It acts as anti-metabolites with side effects mouth sores, diarrhea, vomiting, nausea, appetite loss, sunburn of skin, fever etc.
Plerixafor [100] was approved in 2008 for the treatment of non-Hodgkin lymphoma and multiple myeloma. It acts as immunostimulant for mobilize hematopoietic stem cells in cancer patients with side effects diarrhea, nausea, headache, pain in muscles, dizziness etc.
i) Multiple nitrogen containing synthetic medicines (Table 27)
Goserelin [101] was approved in 1989 and used for the treatment of prostate and breast cancers. It is a decapeptide hormone also called luteinizing hormone releasing hormone (LHRH). It acts gonadotropin releasing hormone super agonist (block the formation of testosterone and estrogen) with side effects swelling, decease in breast size, skin disorder, depression, vaginal dryness, hot flashes, stooping menstrual period etc.
Leuprolide [102] was approved in 1985 and used for the treatment of prostate cancer. It is also decapeptide hormone (a luteinizing hormone releasing hormone). It also acts similar to goserelin as well as similar side effects.
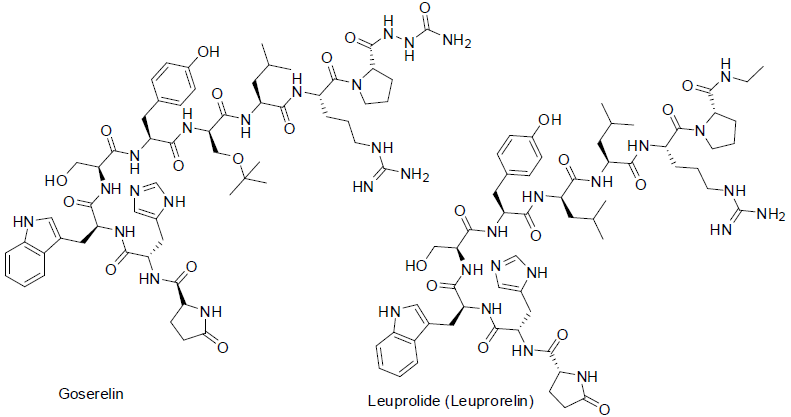
Degarelix [103] was approved in 2008 and used for the treatment of prostate cancer. It is also a decapeptide hormone. It acts gonadotropin releasing hormone super agonist with side effects sleeping disorder, hot flashes, breast enlargement, pain in breast, back pain, headache, constipation, tiredness, increase urination etc.
Ibritumomab tiuxetan [104] was approved in 2002 and used for the treatment of follicular B‑cell non‑Hodgkin’s lymphoma. It is a monoclonal antibody drugs. It acts as CD20 antigen inhibitor with side effects nausea, tiredness, fever, low white blood cell count, low platelet count, low red blood cell count.
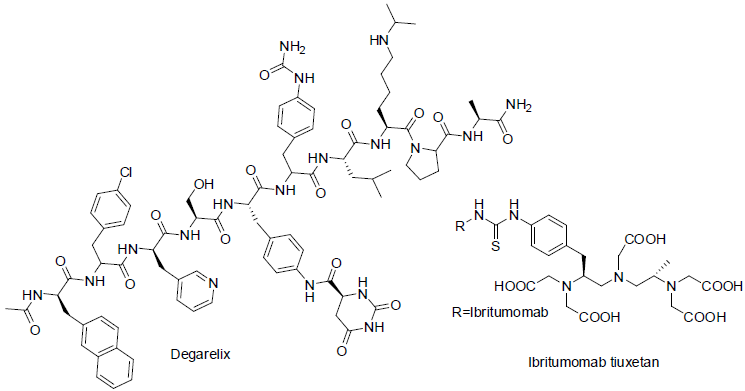
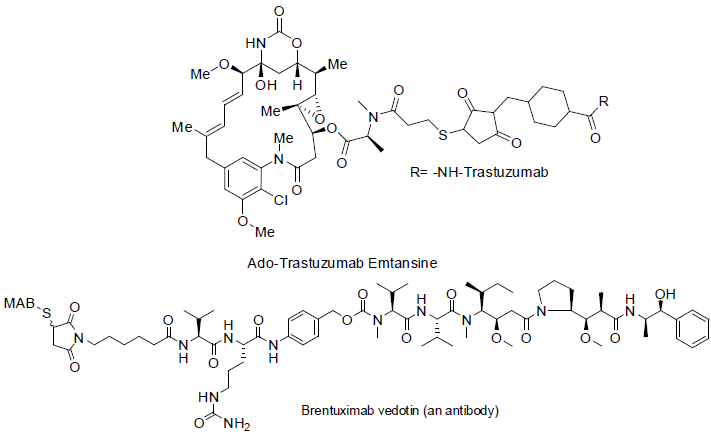
Trastuzumab Emtansine [105] was approved in 2013 and used for the treatment of breast cancer. It contains monoclonal antibody part trastuzumab and second part is emtansine. Transtuzumab inhibit to grow cancer cell binding from HER2/neu receptor and emtansine bind with tubulin. It has found side effect nausea, tiredness, pain in joint, bone and muscles, diarrhea, constipation, headache, low blood platelet count, nose bleeding, numbness etc.
Brentuximab vedotin [106,107] was approved in 2011 and used for the treatment of Hodgkin lymphoma and anaplastic large cell lymphoma (a non-Hodgkin lymphoma). It is also consist of two parts, first part is a monoclonal antibody which inhibit CD30 antigen of carcinoma cell and second part is monomethyl auristatin E (MMAE) which bind with microtubule of carcinoma cells. It has shown side effects tiredness, rashes, cough, fever, nausea, vomiting, diarrhea, numbness in hands, abdomen pain etc.
Without nitrogen containing synthetic medicines (Table 28)
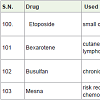
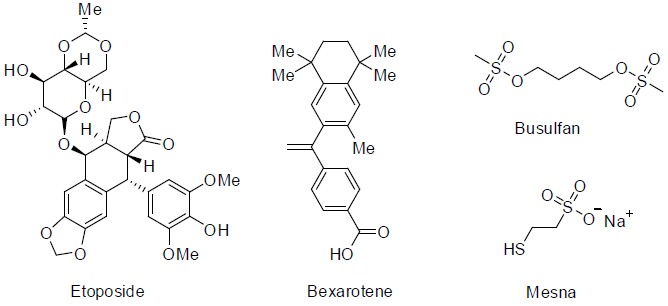
Etoposide [108] was approved in 1983 and used for the treatment of small cell lung and testicular cancer. It acts as topoisomerase II enzyme inhibitors with side effects hair loss, appetite loss, vomiting, nausea, low white blood cell count, low blood platelet count etc.
Bexarotene [109] was approved in 1999 and used for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma and also for lung and breast cancer. It is aretenoid and acts as retinoic acid receptors with side effects diarrhea, headache, rashes, itching, low cholesterol disorder, high lipid levels, pain etc.
Busulfan [110] was approved in 1999 and used for the treatment of chronic myelogenous leukemia (a white blood cell carcinoma). It acts as alkylating agents with side effects low platelet count, low white blood cell count, anemia, hair loss, infertility disorder etc.
Mesna [111] was approved in 1988 and used for the risk reduction of bladder damage during chemotherapy drugs treatment of patients.
Steroidal medicines without nitrogen (Table 29)
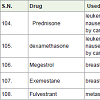
Prednisone and dexamethasone [112] is used for the treatment of leukemias and lymphomas as well as treatment of allergic reactions, treatment of nausea and vomiting, increase of appetite caused by various carcinoma drugs. It are a glucocorticosteroid hormones and have shown side effects sleeping disorder, stomach problem, weight gain, high blood glucose level etc.
Megestrol [113] is used for the treatment of breast and endometrial carcinoma. It acts as hormone antagonists with side effects such as weight gain, increase in appetite, swelling in hands, legs and feet etc.
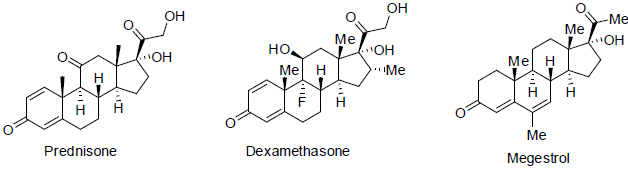
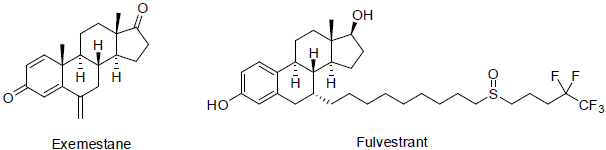
Exemestane [114] was approved in 1999 and used for the treatment of breast cancer. It acts as aromatase inhibitors (block the formation of estrogen by inhibit aromatose) with a side effects such as pain in join and bone, hot flashes, tiredness, headache etc.
Fulvestrant [115] was approved in 2002 and used for the treatment of metastatic breast cancer. It acts as estrogen receptor antagonist with side effects nausea, weight gain, tiredness, vomiting etc.
Other Protein, antibody and amino acids as anticancer medicines (Table 30)
Interleukin 2 (Aldesleukin) [116] was approved in 1992 and used for the treatment of skin melanomas and kidney carcinoma. It is an interleukin (a cytokine signaling molecule in the immune system). It belongs to cytokines protein group and acts as enhancement of immune system for the fight of cancers with side effects fever, headache, pain in joint and muscles, low blood pressure, rashes, weakness, breath problem, confusion, itching in skin, nausea, vomiting, diarrhea etc.
Alemtuzumab [117] was approved in 1992 and used for the treatment of chronic lymphocytic leukemia, cutaneous T-cell lymphoma, T-cell lymphoma, multiple sclerosis as lemtrada, regimens for bone marrow, kidney and islet cell transplantation. It is a monoclonal antibody and bind with CD52 protein of carcinoma cells. It has shown side effects vomiting, fever, nausea, anemia, low white blood cell and platelet count, allergic reactions etc.
Ofatumumab [118] was approved in 2009 and used for the treatment of chronic lymphocytic leukemia. It is a fully human monoclonal antibody and bind with CD20 protein of carcinoma cells to inhibit early-stage B lymphocyte activation with side effects fever, low white blood cell count, lung infection, allergies, tiredness etc.
Bevacizumab [119] was approved in 2004 and used for the treatment of colon, cervical, kidney, breast, brain, non small cell lung cancer. It is also a human monoclonal antibody and acts as angiogenesis inhibitor which inhibit vascular endothelial growth factor (VEGF) protein of carcinoma cells with side effects headache, mouth sores, diarrhea, tiredness, weaknes, back pain, skin dryness, appetite loss, nose bleeding, high blood pressure, bleeding form rectum, red and scaly skin etc.
Cetuximab [120] was approved in 2004 and used for the treatment of head and neck cancer, metastatic colorectal cancer and metastatic non-small cell lung cancer. It is also a human monoclonal antibody and acts as angiogenesis inhibitor which inhibit vascular endothelial growth factor (VEGF) protein of carcinoma cells with side effects itching, mouth sores, tiredness, weakness, rashes, fever etc.
Ramucirumab [121] was approved in 2014 and used for the treatment of stomach cancer. It is also a human monoclonal antibody and acts as angiogenesis inhibitor which inhibits vascular endothelial growth factor (VEGF) protein of carcinoma cells.
Denileukin diftitox [122] was approved in 1999 and used for the treatment of non-Hodgkin lymphoma (cutaneous T-cell lymphoma). It is a combined 2 fusion protein of diphtheria toxin and interleukin-2 and acts CD25 (IL 2 receptor protein) inhibiter and diphtheria toxin inhibit the formation of new carcinoma cell with side effects chills, fever, rashes, weakness, appetite loss, vomiting, nausea, low blood pressure etc.
Denosumab [123] was approved in 2010 and used for the treatment of multiple myeloma in bones. It is also a human monoclonal antibody and acts as osteoclasts inhibitor with side effects diarrhea, nausea, weakness, tiredness, breathe disorder etc.
Liposomal cytarabine [124] was approved in 1999 and used for the treatment of lymphoma which spread from brain or spinal card. It is a form of cytarabine contained liposomes. It acts as anti-metabolites with side effects fever, nausea, vomiting, diarrhea, headache, back pain, sleepiness, weakness etc.
Rasburicase [125] was approved in 1999 and used for the treatment of tumor lysis syndrome (a metabolic complication after treatment of cancer drugs). It is a tetrameric protein and acts as urate oxidase (metabolizes uric acid to allantoin) with side effects fever, mouth sores, diarrhea, headache etc.
Filgrastim [126] was approved in 1999 and used for the treatment of neutropenia (increase white blood cell) in cancer patients. It is a protein called granulocyte colony-stimulating factor (G-CSF).
Obinutuzumab [127] was approved in 1999 and used for the treatment of chronic lymphocytic leukemia. It is a humanized monoclonal antibody and bind with CD20 protein of carcinoma cells and inhibits B-cell (B lymphocytes) with side effects low white blood cell count, fever etc.
Gemtuzumab ozogamicin [128] was approved for 2000-2010 and used for the treatment of acute myelogenous leukemia. It is a monoclonal antibody and bind with CD33 protein of carcinoma cells.
Glucarpidase [129] was approved in 2012 and used for the treatment of toxic plasma methotrexate in cancer patients.
Romiplostim [130] was approved in 2003 and used for the treatment of blood platelet regulation. It is a fusion protein hormone analog of thromboprotein.
Peginterferon alfa-2b [131] was approved in 2011 and used for the treatment of melanoma with side effects such as abdominal pain, chills, fever, cough, depression, nausea, vomiting, etc.
Rituximab [132] was approved in 1997 and used for the treatment of chronic lymphocytic leukemia. It is a chimeric (fusion of human and mouse antibody) monoclonal antibody and bind with CD20 protein of carcinoma cells with side effect headache, fever, nausea, etc.
Siltuximab [133,134] was approved in 2014 and used for the treatment of prostrate cancer, renal cancer and Castleman disease. It is a chimeric (fusion of more than one gene of separated proteins) monoclonal antibody and bind with interleukin-6 protein of carcinoma cells with side effect rashes, weight gain, infections in respiratory system, etc.
Panitumumab [134] was approved in 2006 and used for the treatment of colorectal cancer. It is a fully human monoclonal antibody and bind with epidermal growth factor receptor (EGFR) protein of carcinoma cells with side effect diarrhea, tiredness, abdomen pain, constipation, etc.
Ziv-Aflibercept [135] was approved in 2012 and used for the treatment of colorectal cancer. It is a recombinant fusion protein and bind with epidermal growth factor receptor (EGFR) protein of carcinoma cells with side effects weight loss, diarrhea, mouth sores, low white blood cell count, appetite loss, tiredness, weakness, low blood platelet count etc.
Ipilimumab [136] was approved in 2011 and used for the treatment of melanoma. It is a is a monoclonal antibody and block CTLA-4 protein of carcinoma cells and increase immune efficacy with side effects tiredness, itching, rashes, diarrhea etc.
Palifermin [137] was approved in 2004 and used for the treatment of mouth sores of cancer patients. It is produced in Escherichia coli and acts as keratinocyte growth factor (KGF) for enhancement damaged epithelial cells of mouth and throat of cancer patient.
Sipuleucel-T [138] was approved in 2010 and used for the treatment of prostate cancer. It is a protein subunit and increase immune system of patient with side effects fever, chill, nausea, weakness, back pain etc.
Enzymes and Vaccine medicines (Table 31)
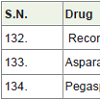
Recombinant Human Papillomavirus [139] was approved in 2006 and used for prevent from cervical, anal, vaginal, vulvar (external genital organs of female) cancers. It is a virus-like particle which is not produce in human body.
Asparaginase Erwinia chrysanthemi [140] was approved in 2011 and used for the treatment of acute lymphoblastic leukemia.
Asparaginase (colaspase) is an enzyme that catalyzes the hydrolysis of asparagines (aspartic acid). Asparaginases are naturally occurringenzymes and produced by microorganisms Erwinia chrysanthemi.
Pegaspargase [141] was approved in 2006 and used for the treatment of acute lymphoblastic leukemia. It is a pegylated E. coli L-asparagine amidohydrolase.
Inorganic medicines (Table 32)
Arsenic Trioxide (As2O3) [142] was approved in 2000 and used for the treatment of leukemia with side effects vomiting, nausea, appetite loss, abdomen pain, headache, tiredness, cough, fever, rashes etc.
Radium223dichloride [143] was approved in 2013 and used for the treatment of prostrate cancer. It is a radio pharma drug and its radiation damaged DNA of cancer cell in body with side effects such as nausea, diarrhea, anemia, low white blood cell and platelet count etc.
Combined drugs (Table 33)
Due to several side effects of a single drug, resistant of drug, unavailability of drugs for advanced carcinona and for a better chemotherapy result combined drugs course are used for the treatment ofcancer. The combined drugs are ABVD (Adriamycin, Bleomycin, Vinblastine, Dacarbazine; used in Hodgkin lymphoma), ABVE (Adriamycin, Bleomycin, Vinblastine, Etopside, used in Hodgkin lymphoma of children), ABVE-PC (ABVE-Prednisone, Cyclophosphamide; used in Hodgkin lymphoma of children), AC (Adriamycin, Cyclophosphamide; used in breast cancer) AC-T(AC-Taxol; used in breast cancer), ADE (Cytarabine, Daunorubicin, Etopside; used for acute myeloid leukemia in children), BEACOPP(Bleomycin, Etopside, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, Prednisone; used in Hodgkin lymphoma), BEP (Bleomycin, Etopside, Cisplatin, used in malignant tumor of ovaries and testis), CAF (Cyclophosphamide, Adriamycin, Fluorouracil; used in breast cancer), CAPOX (Capecitabine, Oxaliplatin; used in Colorectal cancer), CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone; used in non Hodgkin lymphoma), CMF (Cyclophosphamide, Methotrexate, Fluorouracil; used in breast cancer), COPP (Cyclophosphamide, Vincristine, Procarbazine, Prednisone; used in non Hodgkin lymphoma and Hodgkinlymphoma) COPP-ABV (COPP- Adriamycin, Bleomycin, Vinblastine; used in Hodgkin lymphoma), CVP (Cyclophosphamide, Vincristine, Prednisone; used in non Hodgkin lymphoma and Chronic lymphocytic leukemia), EPOCH (Etopside, Prednisone, Vincristine, Cyclophosphamide, Hydroxydaunomycin; used in non Hodgkin lymphoma), FEC (Fluorouracil, Epirubicin,Cyclophosphamide; used in breast cancer), FOLFIRI (Leucovorin, Fluorouracil, Irinotecan; used in colorectal cancer, FOLFOX (Leucovorin, Fluorouracil, Oxaliplatin; used in colorectal cancer), FU-LU (Fluorouracil, Leucovorin; used in colorectal cancer), ICE (Ifosfamide, Carboplatin, Etopside; used in non Hodgkin lymphoma and Hodgkin lymphoma), MOPP (Mechlorethamine, Vincristine, Procarbazine, Prednisone; used in Hodgkin lymphoma), OEPA (Vincristine, Etopside, Prednisone, Adriamycin; used in Hodgkin lymphoma), OFF (Oxaliplatin, Fluorouracil, Leucovorin; used in pancreatic cancer), OPPA (Vincristine, Procarbazine, Prednisone, Adriamycin, Dexamethasone; used in multiple myloma), R-CHOP(Rituximab-CHOP; used in non Hodgkin lymphoma), R-CVP (Rituximab-CVP; used in non Hodgkin lymphoma), STANFORD V (Mechlorethamine, Doxorubicin, Vinblastine, Bleomycin, Etopside, Prednisone; used in Hodgkin lymphoma), TAC (Docetaxel, Adriamycin, Cyclophosphamide; used in breast cancer), TPF(Docetaxel, Cisplatin, Florouracil; used in gastric and squamous cell carcinoma of the head and neck), VAMP (Vincristine, Adriamycin, Methotrexate, Prednisone; used in Hodgkin lymphoma), XELOX (Capecitabine, Oxaliplatin; used in colorectal cancer).
Conclusion
This review have been covered anticancer drugs classified on the bases of present of nitrogen in the anticancer drugs with their structure, actions on cancer cell and side effects, the results are showing that these medicine are effecting cancer patient with a serious side effects. These side effects are itself a disease and several times these side effects became a main reason for a patient death. Thus, it needs new efforts with new thoughts to develop an anticancer medicine without side effects as well as cost effective medicine. Classification shown that four and three nitrogen containing anticancer are available in greater number as comparison of others.
Acknowledgement
This review is dedicated to my maternal grandfather (Let. Sohan Lal), his elder brother, my favorite teacher and an aunt. They weredied after suffering from deadly side effects of cancer drugs for morethan six month treatment. I am also acknowledged DST, New Delhi, India for the awarded of DST fast track young scientist fellowship.
References
- http://www.medicalnewstoday.com
- www.cancer.org
- www.cancer.gov
- Sigel RS, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9-29.
- World Health Organization report 2013, press release No 223, 12 December 2013.
- Ali I, Wani WA, Saleem, K (2011) Cancer Scenario in India with Future Perspectives. Cancer therapy 8: 56-70.
- Casinelli G, Orezzi P (1963) Daunomycin, a new antibiotic with cytostatic activity-isolation and properties. Giorn Microbiol 11: 167-174.
- Arcamone F, Cassinelli G, Orezzi P, Franceschi G, Mondelli R (1964) Daunomycin-II. The structure and stereochemistry of daunosamine. J Am Chem Soc 86: 5335-5336.
- Di Marco A, Gaetani M, Dorigotti L, Soldati M, Bellini, O (1964) Daunomycin-A new antibiotic with antitumor activity. Cancer Chemother Rep 38: 31-38.
- Brayfield A ed. (2013) "Doxorubicin" Martindale: The Complete Drug Reference, Pharmaceutical Press, Retrieved 15 April 2014.
- Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharmac Pharmaco 65: 157-170.
- Bonfante V, Bonadonna G, Villani F, Martini A (1980) Preliminary clinical experience with 4-epidoxorubicin in advanced human neoplasia. Recent Results Cancer Res 74: 192-199.
- Goodman J, Walsh V. (2001) The Story of Taxol: Nature and politics in the pursuit of an anti-cancer drug, Cambridge University Press: 179-182.
- Lyseng-Williamson KA, Fenton C (2005) Docetaxel: a review of its use in metastatic breast cancer. Drugs 65: 2513-2531.
- Galsky MD, Dritselis A, Kirkpatrick P, oh WK (2010) Cabazitaxel. Nat Rev Drug Discov 9: 677-678.
- Ma WW, Jimeno A (2007) Temsirolimus. Drugs Today 43: 659-669.
- Wachowska M, Muchowicz A, Firczuk M, Gabrysiak M, Winiarska M, et al. (2011) Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 16: 4140-4164.
- Nashan B (2002) Review of the proliferation inhibitor everolimus. Expert Opin Investig Drugs11: 1845-1857.
- Huyck TK, Gradishar W, Manuguid F, Kirkpatrick P (2011) Eribulin mesylate. Nat Rev Drug Discov 10: 173-174.
- Wetzler M, Segal D (2011) Omacetaxine as an anticancer therapeutic: What is old is new again. Curr Pharm Design 17: 59-64.
- Kim TD, Frick M, Coutre P le (2011) Omacetaxine mepesuccinate for the treatment of leukemia. Expert Opin Pharmaco 12: 2381-2392.
- Conlin A, Fornier M, Hudis C, Kar S (2007) Ixabepilone. Nat Rev Drug Discov 6: 953-954.
- 23.Johnson IS, Armstrong JG, Gorman M, Burnett JP (1963) The vinca alkaloids: a new class of oncolytic agents. Cancer Res 23: 1390-1427.
- Li Q, Zu Y, Shi RZ, Yao, LP (2006) Review camptothecin: current perspectives. Curr Med Chem 13: 2021-2039.
- Tomasz M (1995) Mitomycin C: small, fast and deadly (but very selective). Chem Biol 2: 575-579.
- Bertino EM, Otterson GA, (2011) Romidepsin: a novel histone deacetylase inhibitor for cancer. Expert Opin Invest Drug. 20: 1151-1158.
- Steele JM (2013) Carfilzomib: A new proteasome inhibitor for relapsed or refractory multiple myeloma. J Oncol Pharm Pract 19: 348-354.
- Hollstein U (1974) Actinomycin. Chemistry and mechanism of action. Chem Rev 74 : 625-652.
- Blum RH, Carter SK, Agre K (1973) A clinical review of bleomycin-a new antineoplastic agent. Cancer 31: 903-914.
- Froudarakis M, Hatzimichael E, Kyriazopoulou L, Lagos K, Pappas P, et al. (2013) Revisiting bleomycin from pathophysiology to safe clinical use. Crit Rev Oncol Hematol 87: 90-100.
- Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, et al. (2000) Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 343: 1750-1757.
- Gilman A (1963) The initial clinical trial of nitrogen mustard. Am J Surg 105: 574-578.
- Logothetis CJ, Efstathiou E, Manuguid F, Kirkpatrick P (2011) Abiraterone acetat. Nat Rev Drug Discov 10: 573-574.
- Jordan VC (2006) Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 147: 269-276.
- Vogel CL, Johnston MA, Capers C, Braccia D (2014) Toremifene for breast cancer: A review of 20 years of data. Clin Breast Cancer 14: 1-9.
- Hansdóttir H (2008) Raloxifene for older women: a review of the literature. Clin Interv Aging 3: 45-50.
- Sorrentino MF, Kim J, Foderaro AE, Truesdell AG (2012) 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J 19: 453-458.
- Likun Z, Xiang J, Yi B, Xin D, Tao ZL (2011) A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist 16: 207-216.
- Goa KL, Spencer CM. (1998) Bicalutamide in advanced prostate cancer. A review. Drug Aging 12: 401-422.
- Dlugosz A, Agrawal S, Kirkpatrick P (2010) Vismodegib. Nat Rev Drug Discov 11: 437-438.
- Stillwell TJ, Benson RC, (1988) Cyclophosphamide-induced hemorrhagic cystitis: A review of 100 patients. Cancer 61: 451-457.
- Tascilar M, Loos WJ, Seynaeve C, Verweij J, Sleijfer S (2007) The pharmacologic basis of ifosfamide use in adult patients with advanced soft tissue sarcomas. Oncologist 12: 1351-1360.
- Teni B, Maria V (2003) Cisplatin and platinum drugs at the molecular level (Review). Oncology 10: 1663-1682.
- Misset JL, Bleiberg H, Sutherland W, Bekradda M, Cvitkovic E (2000) Oxaliplatin clinical activity: a review. Crc Cr Rev Oncol Hem 35: 75-93.
- Go RS, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17: 409-422.
- Rajkumar SV, Witziq TE (2000) A review of angiogenesis and antiangiogenic therapy with thalidomide in multiple myeloma. Cancer Treat Rev 26: 351-362.
- Grant S, Easley C, Kirkpatrick P (2007) Vorinostat. Nat Rev Drug Discov 6: 21-22.
- Reese ND, Schiller GJ (2013) High-Dose Cytarabine (HD araC) in the Treatment of Leukemias: a Review. Curr Hematol Malig Rep 8: 141-148.
- Noble S, Goa KL (1997) Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 54: 447-472.
- Prados MD, Scott C, Curran Jr WJ, Nelson DF, Leibel S, et al. (1999) Procarbazine, Lomustine, and Vincristine (PCV) Chemotherapy for Anaplastic Astrocytoma: A Retrospective Review of Radiation Therapy Oncology Group Protocols Comparing Survival With Carmustine or PCV Adjuvant Chemotherapy. J Clini Oncol 17: 3389-3395.
- Keating MJ, Bach C, Yasothan U, Kirkpatrick P (2008) Bendamustine Nat Rev Drug Discov 7: 473-474.
- Nagilla M, Brown RL, Cohen, EEW (2012) Cabozantinib for the Treatment of Advanced Medullary Thyroid Cancer. Adv Ther 29: 925-934.
- Walko CM, Lindley C (2005) Capecitabine: A review. Clin Ther 27: 23-44.
- Tang PA, Tsao M-S, Moore MJ (2006) A review of erlotinib and its clinical use Expert Opin. Pharmaco 7: 177-193.
- Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, et al. (2012) Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 11: 873-886.
- Ahmad T, Gore M (2004) Review of the use of topotecan in ovarian carcinoma. Expert Opin Pharmaco 5: 2333-2340.
- Weiss RB, Issell BF (1982) The nitrosoureas: carmustine (BCNU) and lomustine (CCNU) Cancer Treat. Rev. 9:313-310.
- 458.Hideshima T, Raje N, Anderson KC (2008) A review of lenalidomide in combination with dexamethasone for the treatment of multiple myeloma. Ther Clin Risk Mang 4: 129-136.
- Scott LJ (2014) Pomalidomide: a review of its use in patients with recurrent multiple myeloma. Drugs 74: 549-562.
- Vismans JJ, Briët E, Meijer K, den Ottolander GJ (1980) Azathioprine and Subacute Myelomonocytic Leukemia. Acta Med Scand 207: 315-319.
- Miller RL, Gerster, JF, Owens, ML, Slade HB, Tomai MA (1999) Review Article Imiquimod applied topically: a novel immune response modifier and new class of drug. International J Immunopharmacology 21: 1-14.
- Derissen EJ, Beijnen JH, Schellens JH (2013) Concise drug review: azacitidine and decitabine. Oncologist 18: 619-624.
- Curran MP (2013) Decitabine: A Review of its Use in older patients with acute myeloid leukaemia. Drugs Aging 30: 447-458.
- Chen X, Liu Y, Qian Y, Guo R, Zhu L, et al. (2013) Gefitinib or Erlotinib as maintenance therapy in patients with advanced stage non-small cell lung Cancer: A systematic review. PLos One.
- Moy B, Kirkpatrick P, Kar S, Goss P (2007) Lapatinib. Nat Rev Drug Discov 6: 431.
- Karras S, Anagnostis P, Krassas GE (2014) Vandetanib for the treatment of thyroid cancer: an update Expert Opin Drug Met 10: 469-481.
- Van Geel RMJM, Beijnen JH, Schellens JHM (2012) Concise drug review: pazopanib and axitinib. Oncologist 17: 1081-1089.
- Iyer R, Fetterly G, Lugade A, Thanavala Y (2010) Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmaco 11: 1943-1955.
- Davis SL, Eckhardt SG, Messersmith WA, Jimeno A (2013) The development of regorafenib and its current and potential future role in cancer therapy. Drugs Today 49: 105-115.
- Motzer RJ, Hoosen S, Bello CL, Christensen JG (2006) Sunitinib malate for the treatment of solid tumors: a review of current clinical data. Expert Opin Investig Drugs 15: 553-561.
- Dando TM, Perry CM (2004) Aprepitant: a review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 64: 777-794.
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, et al. (2007) Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 357: 2237-2247.
- Richardson PG (2004) A review of the proteasome inhibitor bortezomib in multiple myeloma. Expert opin Pharmaco 5: 1321-1331.
- Cvetković RS, Scott LJ (2005) Dexrazoxane : a review of its use for cardioprotection during anthracycline chemotherapy. Drugs 65:1005.
- Sanford M (2013) Enzalutamide: A review of its use in metastatic, castration-resistant prostate cancer. Drugs 73: 1723-1732.
- Bhatnagar AS (2006) Review of the development of letrozole and its use in advanced breast cancer and in the neoadjuvant setting. Breast 15: 3-13.
- Pazâ€Ares L, Bezares S, Tabernero JM, Castellanos D (2003) Review of a promising new agent-pemetrexed disodium. Cancer 97: 2056-2063.
- Dando TM, Perry CM (2004) Aprepitant: a review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 64: 777-794.
- Gandhi V, Keating MJ, Bate G, Kirkpatrick P (2006) Nelarabine. Nat Rev Drug Discov 5:17-18.
- Ricci F, Tedeschi A, Morra E, Montillo M (2009) Fludarabine in the treatment of chronic lymphocytic leukemia: a review. Ther Clin Risk Manag 5: 187-207./a>
- Kline JP, Larson RA (2005) Clofarabine in the treatment of acute myeloid leukaemia and acute lymphoblastic leukaemia: a review. Expert Opin Pharmacother 6: 2711-2718.
- Keating GM (2014) Afatinib: A review of its Use in the Treatment of Advanced Non-Small Cell Lung Cancer. Drugs 74: 207-221.
- Rusconi F, Piazza R, Vagge E, Gambacorti-Passerini C (2014) Bosutinib: a review of preclinical and clinical studies in chronic myelogenous leukemia. Expert Opin Pharmaco 15: 701-710.
- Davis JE, Anderson JE, Kim KB (2014) Dabrafenib therapy for advanced melanoma. Ann Pharmacother 48: 519-529.
- Cooper MR, Chim H, Chan H, Durand, C (2014) Ceritinib a new tyrosine kinase inhibitor for NSCLC. Ann Pharmacother [Epub ahead of print].
- Salama AKS, Kim KB (2013) Trametinib (GSK1120212) in the treatment of melanoma. Expert opin Pharmaco 14: 619-627.
- Shaw AT, Yasothan U, Kirkpatrick P (2011) Crizotinib. Nat Rev Drug Discov 10: 897-898.
- McDermott J, Jimeno A (2014) Ibrutinib for the treatment of chronic lymphocytic leukemia and mantle cell lymphoma. Drugs Today 50: 291-300.
- Hoy SM (2014) Ponatinib: a review of its use in adults with chronic myeloid leukaemia or philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs 74: 793-806.
- Swaim SJ (2014) Ruxolitinib for the treatment of primary myelofibrosis. Am J Health Syst Pharm 71: 453-462.
- Wilson KS (2011) Ipilimumab plus dacarbazine in melanoma. N Engl J Med 365: 1256.
- Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23: 35-61.
- Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P (2006) Dasatinib. Nat Rev Drug Discov 5: 717-718.
- Bukowski RM, Yasothan U, Kirkpatrick P (2010) Pazopanib. Nat Rev Drug Discov 9:17-18.
- Essat M, Cooper K (2011) Imatinib as adjuvant therapy for gastrointestinal stromal tumors: a systematic review. Int J Cancer 128: 2202-2214.
- Plosker GL, Robinson DM (2008) Nilotinib. Drugs 68: 449-459.
- Hong YS, Nam BH, Kim K, Kim JE, Park SJ, et al. (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet 15: 1245-1253.
- Dondi A, Bari A, Pozzi S, Ferri P, Sacchi F (2014) The potential of pralatrexate as a treatment of peripheral T-cell lymphoma. Expert Opin Invest Drug 23: 711-718.
- Boulter PS (1967) Methotrexate in cancer. Br Med J. Mar 4: 553.
- DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P (2009) Plerixafor. Nat Rev Drug Discov 8: 105-106.
- Perry CM, Brogden RN (1996) Goserelin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in benign gynaecological disorders. Drugs 51: 319-346.
- Mitchell MA (2005) Therapeutic review: Leuprolide acetate. Semin Avian Exot Pet Med 14: 153-155.
- Carter NJ, Keam SJ (2014) Degarelix: a review of its use in patients with prostate cancer. Drugs 74: 699-712./a>
- Gisselbrecht C, Bethge W, Duarte RF, Gianni AM, Glass B, et al. (2007) Current status and future perspectives for yttrium-90 (90Y)-ibritumomab tiuxetan in stem cell transplantation for non-Hodgkin's lymphoma. Bone marrow Transplant 40: 1007-1017.
- Niculescu-Duvaz I (2010) Trastuzumab emtansine, an antibody-drug conjugate for the treatment of HER2+ metastatic breast cancer. Curr Opin Mol Ther 12 : 350-360.
- Younes A, Yasothan U, Kirkpatrick P (2012) Brentuximab vedotin. Nat Rev Drug Discov 11: 19-20.
- Katz J, Janik JE, Younes A (2011) Brentuximab vedotin (SGN-35). Clin Cancer Res.17: 6428-6436.
- Hande KR (1998) Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34: 1514-1521.
- Gniadecki R, Assaf C, Bagot M, Dummer R, Duvic M, et al. (2007). The optimal use of bexarotene in cutaneous T-cell lymphoma. Br J Dermatol 157: 433-440.
- Sostman HD, Putman CE, Gamsu G (1981) Review: diagnosis of chemotherapy lung. Am J Roentgenol 136: 33-40.
- Shaw IC, Graham MI (1987) Mesna-a short review. Cancer Treat Rev 14: 67-86.
- Wooldridge JE, Anderson CM, Perry MC (2001) Corticosteroids in advanced cancer. Oncology 15: 225-234.
- Strang P (1996) The effect of megestrol acetate on anorexia, weight loss and cachexia in cancer and AIDS patients (review). Anticancer Res 17: 657-662.
- Goss PE, Ingle JN, Alés-MartÃnez JE, Cheung AM, Chlebowski RT, et al. (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364: 2381.
- Lee CI, Goodwin A, Freedman O, Clemons M, Wilcken N (2014) Fulvestrant for hormone-sensitive metastatic breast cancer. The Cochrane Library.
- Rosenberg SA (1988) The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg 208: 121-135.
- O'Brien SM, Kantarjian HM, Thomas DA, Cortes J, Giles FJ, et al. (2003) Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer 98: 2657-2663.
- Cheson BD (2010) Ofatumumab, a Novel Anti-CD20 Monoclonal Antibody for the Treatment of B-Cell Malignancies. J Clin Oncol 28: 3525-3530.
- Ferrara N, Hillan KJ, Gerber HP, Novotany W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391-400.
- Merlano M, Occelli M (2007) Review of cetuximab in the treatment of squamous cell carcinoma of the head and neck. Ther Clin Risk Manag 3: 871-876.
- Walker T (2014) FDA approves advanced stomach cancer drug.
- Prince HM, Newland KM (2014) Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Expert Opin. Orphan Drug 2: 625-634.
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, et al. (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29: 1125-1132.
- Benesch M, Urban C (2008) Liposomal cytarabine for leukemic and lymphomatous meningitis: recent developments. Expert Opin Pharmacother 9: 301-309.
- Lopez-Olivo MA, Pratt G, Palla SL, Salahudeen A (2013) Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis. Am J Kidney Dis 62: 481-492.
- Sourgens H, Lefrère F (2011) A systematic review of available clinical evidence-filgrastim compared with lenograstim. Int J Clin Pharmacol Ther 49: 510-518.
- Illidge TM (2012) Obinutuzumab (GA101)-a different anti-CD20 antibody with great expectations. Expert opin Biol Ther 12: 543-545.
- Kharfanâ€Dabaja MA, Hamadani M, Reljic T, Pyngolil R, Komrokji RS, et al. (2013) Gemtuzumab ozogamicin for treatment of newly diagnosed acute myeloid leukaemia: a systematic review and meta-analysis. Brit J Haematol 163: 315-325.
- Cada DJ, Demaris K, Levien TL, Baker DE (2012) Glucarpidase. Hosp Pharm 47: 463.
- Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, et al. (2010) Romiplostim or Standard of Care in Patients with Immune Thrombocytopenia. Engl J Med 363: 1889-1899.
- Eggermont AMM, Spatz A, Robert C (2014) Cutaneous melanoma. Lancet 383: 816-827.
- Gürcan HM, Keskin DB, Stern JNH, Nitzberg MA, Shekhani H, et al. (2009) A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9: 10-25.
- Markham A, Patel T (2014) Siltuximab: first global approval. Drugs 74: 1147-1152.
- Tol J, Punt CJA (2010) Monoclonal antibodies in the treatment of metastatic colorectal cancer: A review. Clin Ther 32: 437-453.
- Mitchell EP (2013) Targeted therapy for metastatic colorectal cancer: role of aflibercept. Clin colorectal can 12: 73-85.
- Sondak VK, Smalley KSM, Kudchadkar R, Grippon S, Krikpatrick P (2011) Ipilimumab. Nat Rev Drug Discov 10: 411-412.
- Beaven AW, Shea TC (2007) The effect of palifermin on chemotherapy and radiation therapy-induced mucositis: a review of the current literature. Support Cancer Ther 4: 188-197.
- Quinn DI, Vaishampayan U, Higano CS, Lin DW, Shore ND, et al. (2014) Sequencing therapy in advanced prostate cancer: focus on sipuleucel-T. Expert rev anticanc Ther 14: 51-61.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, et al. (2014) Human papillomavirus vaccination. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports. 63: 1-30.
- Salzer WL, Asselin BL, Plourde PV, Corn T, Hunger SP (2014) Development of asparaginase Erwinia chrysanthemi for the treatment of acute lymphoblastic leukemia. Ann N Y Acad Sci 1329: 81-92.
- Chang J, Zhang Y, Feng L, Zhong X, Wang L (2014) Treatment of Children with Advanced-Stage Lymphoblastic Lymphoma with Pegaspargase. Iran J Pediatr 24: 75-80.
- Sanz MA, Fenaux P, Coco FL (2005) Arsenic trioxide in the treatment of acute promyelocytic leukemia. a review of current evidence. Haematologica 90: 1231-1235.
- Shirley M, McCormack PL (2014) Radium-223 Dichloride: A Review of Its Use in Patients with Castration-Resistant Prostate Cancer with Symptomatic Bone Metastases. Drugs 74: 579-586.