Review Article
History of Tissue Engineering and Regeneration with Modern Day Applications in Medicine
Tanyi EO*
Wake Early College of Health & Sciences, Raleigh, NC 27603, USA
*Corresponding author: Tanyi EO, Wake Early College of Health & Sciences, Raleigh, NC 27603, USA;
E-mail: eotanyi@waketech.edu
Copyright: © 2023 Tanyi EO. This is an open access article distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 03/01/2023; Accepted: 24/02/2023; Published: 28/02/2023
Abstract
Regenerative medicine is a scientific process of growing living, functional tissues to repair or replace tissue or organ function that has been depleted
due to age, disease, injury, or congenital defects. Regenerative medicine can also be referred to as a group of biomedical approaches to clinical therapies
that may involve the use of stem cells. This branch in medicine enables scientists to grow tissues and organs in the laboratory which could be surgically
implanted into specific locations in the human body to progressively heal itself to restore depleted organs function. Regenerative medicine has the potential
to solve broad ranged medical issues like shortage of organs available for donation compared with the number of patients that require them. Furthermore,
it could be used in transplantation, in particular, in complex organ transplant rejection which is slowly but surely being pedestaled for the common-sense
practical reason that the organ’s cells must match that of the patient. Examples include injection of stem cells or progenitor cells (i.e., cell therapies); induction
of regeneration by biologically active molecules (i.e., immunomodulation); and transplantation of in vitro grown organs and tissues (i.e., tissue engineering).
Abbreviations
ASC’s: Adult Stem Cells, STAP cells: Stimulus-triggered Acquisition of Pluripotency Cells, iPSC’s: induced Pluripotent Cells, ESC’s:
Embryonic Stem Cells, SCNT: Somatic Cell Nuclear Transfer, CSSC’s: Corneal Stromal Stem Cells, LESC’s: Limbal Epithelial Stem Cells, PSC’s: Pluripotent
Stem Cells, NIH: National Institutes of Health, NHGRI: National Human Genome Research Institute, NIBIB: National Institute of Biomedical Imaging &
Bioengineering
Introduction
Now patients with diseased and injured organs are treated with
transplanted organs when deemed appropriate. Nevertheless, there is
a shortage of donor organs due to the aging population compounded
by a stark rise in the number of new cases of organ failure [1]. Due
to the limited number of organs, scientists are forced to rely on other
innovative approaches such as the principles of cell transplantation,
material science and bioengineering to construct biological
silhouettes capable of replicating and restoring normal function of
affected tissues [1]. Stem cell harvesting is a rapidly advancing part
of regenerative medicine leading to new discoveries and novel stem
cells, such as amniotic fluid and placental stem cells that can avoid the
ethical dilemma associated with embryonic stem cells [1].
The processes of therapeutic cloning and creation of induced
pluripotent cells (iPSC’s) provides potential sources of stem cells
desperately needed for cell-based tissue engineering applications.
Consensus about stem cells still being in the rudimentary research
phase is widely acknowledged but some tissue engineering therapies
involving autologous and adult stem cells have breached numerous
clinical settings. This clearly signals the promise and scope of
regenerative medicine for futuristic medical and pharmaceutical
therapies is humongous [1].
An Elaborate historical timeline from 600 BC to 2016 AD:
Ancient practice of surgery in India, 600 BC: Ancient
documented texts of surgery and anatomy in south Asia were given the titles of Sushruta Samhita (6th century BC) and Charaka samhita
(4th century BC) with various methods to repair torn earlobes with
cheek skin and reconstruction of nose from flap of forehead skin
[2]. The important information mentioned in both references was
sourced through animal sacrifice, improperly buried human bodies
and direct patient examination as depicted in Figure 1 [3].
Figure 1: Sushruta is shown examining a patient’s radial pulse & clinic
workers are shown compounding various types of medications [3].
Freshwater Hydra regeneration discovered, 1740: A research uncovered how hydra regrows lost heads, its ability to regenerate and
constantly replace damaged body parts, replace all the cells every 20
days and 27,000 genes that play a critical role in these transformative
biological processes [4].
Demonstration of artificial embryo twinning in Sea urchins, 1885: The first ever significant demonstration of cloning was
performed by a German philosopher and biologist Hans Dreish, by
shaking a 2 celled sea urchin embryo to split the cells and proved that
each individual cell grew into a healthy adult sea urchin. Therefore,
clearly determines that each cell has its set of genetic information [5].
Starfish regeneration, 1901: Regeneration (originally published
in 1901) was a path-breaking book developed by putting together a
series of lectures delivered by Thomas H. Morgan on embryology
and a diversity of organisms that had the innate ability to regenerate
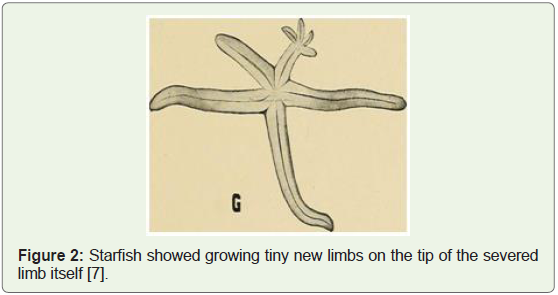
along with experimental evidence (e.g. starfish diagram shown below
in Figure 2 regenerating 4 new limbs from the tip of a severed limb).
He argued that regeneration was a fundamental aspect of the growth
process of an individual organism [6].
Figure 2: Starfish showed growing tiny new limbs on the tip of the severed
limb itself [7].
Embryo cloning in a Vertebrate, 1902: Hans Spemann separated
two salamander embryos at a very early stage and they both grew into
healthy adult salamanders. This experiment also proved that if the
embryo had already matured, then cloning of the same would not
have been possible [8].
Frog embryonic cells grown in lab, 1907: Embryologist Ross
Harrison was credited for developing the first technique of cell
culture in vitro in 1907 at Yale University where small pieces of living
frog embryonic tissue were isolated and successfully grown outside
the body [9].
Salamander embryos developed from cell nucleus experiment, 1928: Spemann continued his lab experiment on salamanders and
pushed the nucleus of a fertilized egg to one side of the cytoplasm
and after four cell divisions, resulted in 16 cells. The same nucleus
was allowed to slide back into the non-dividing side of the egg which
resulted in 16 separate salamander embryos [10].
Successful nuclear transfer from tadpole embryo, 1952: Robert
Briggs and Thomas King proved that the nucleus derived from an
early embryo into a nucleus deprived frog egg resulted in a developed
tadpole. This established that nuclear transfer was a viable cloning
technique and reinforced that it is the nucleus that directs cell growth
and also early embryonic cells are preferred for cloning [11].
Nuclear transfer from tadpole intestinal cells, 1958: John
Gurdon reported growing adult South African clawed toads after
transferring nuclei from tadpole intestinal epithelial cells. He also
observed that the same was not possible when intestinal cells were
isolated from adult frogs of the same species [12].
Cloning via somatic cell nuclear transfer succeeds, 1962: A scientifically developed method termed as somatic cell nuclear
transfer (SCNT) was found to confer totipotency; defined as the
ability of a cell to give rise to all cell types of an entire organism.
John Gurdon demonstrated the same by isolating differentiated frog
somatic cells [13].
Self-renewing stem cells in mouse bone marrow, 1963: Toronto
researcher’s duo Jim Till and Ernest McCulloch were the first to
observe hematopoietic stem cells in mouse bone marrow and proved
their existence via a series of lab experiments [14]. This research laid
the foundation for further studies to isolate, analyze characteristics
and develop stem cell applications in medicine [15].
Nuclear transfer rabbit embryo successful, 1975: First scientist
to report developing a rabbit embryo in vitro was Derek Bromhall.
This was achieved by transferring rabbit nuclei into nucleus free
rabbit eggs in spite of the smaller size of mammalian somatic cells and
challenges that come with microscopic level lab experiments [11].
Lab grown embryonic mouse stem cells & lab skin heals wounds, 1981: Gail Martin grew embryonic stem cells in the lab
medium by isolating single cells of mice. These were later harvested
to conclusively demonstrate the pluripotency by observation of these
very same cell lines that grew into a wide variety of cell types [16].
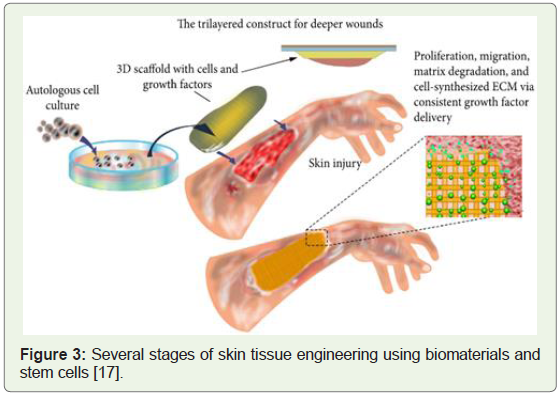
In a separate research study conducted in the same year, cells
isolated from skin biopsies stem cells were derived and clinically
applied to treat severe wounds (Figure 3) [18].
Figure 3: Several stages of skin tissue engineering using biomaterials and
stem cells [17].
Nuclear transfer creation of first mammal, 1984: Research work
by Steen Willadsen resulted in the very first normal cloned lambs
being born [19]. This was achieved by nuclear transfer from an eight
celled lamb embryo that was the cornerstone for further research due
to the possibility of clones from single embryos of other farm animals.
The initial experiments in mammals that inspired sheep cloning were
earlier conducted by Solter and McGrath by applying nuclear transfer
methods to mouse embryos [20].
Nuclear transfer from an embryonic cell to produce calves, 1987: The first team of dairy scientists to achieve success by nuclear
cell transfer into a bovine embryo was Prather et al [21]. Although
only 2 healthy live calves were born out of 19 bovine embryos that
were being studied, the results encouraged further investigation of
nuclear transfer transplants.
Nuclear transfer done from lab cultured sheep cells, 1996: Wilmut and Campbell based in scotland, totally avoided donor
nuclei from cells of early embryos which was the standard norm.
They demonstrated that nuclei from cells that had been isolated from
an adult sheep, transferred and multiplied in a petri dish in the lab
implanted into sheep egg cells resulted in a normal lamb being born
[22].
Somatic cell nuclear transfer mammal created, 1996: Sheep
were the first large animal model in nuclear transfer research projects
and the first mammal to be ever cloned in 1996 by Wilmut and
Campbell. They transferred the nucleus from an adult sheep udder
cell and implanted them into early embryos. Of the 29 early embryos
which developed only one pregnancy went to full term [20].
First primate created from embryonic nuclear transfer, 1997: Meng et al transferred early-stage embryonic cells into monkey egg
cells and the resulting embryos were then implanted into surrogate
mothers out of which 2 rhesus monkeys were born [23]. This
conclusively proved that primates which are human species’s closest
relatives could be cloned.
First transgenic mammal created from genetic engineered lab cells, 1997: Schnieke et al introduced the human factor IX gene, a
plasma protein with a role in blood clotting in humans into the
genome of sheep skin cells grown in a petri dish, to create transgenic sheep. The process involved donor DNA from the cultured transgenic
cells. Of the 7 lambs that were born only one lamb produced factor
IX protein in her milk. This helped to prove that mammals could be
engineered to make therapeutic and other useful proteins [24].
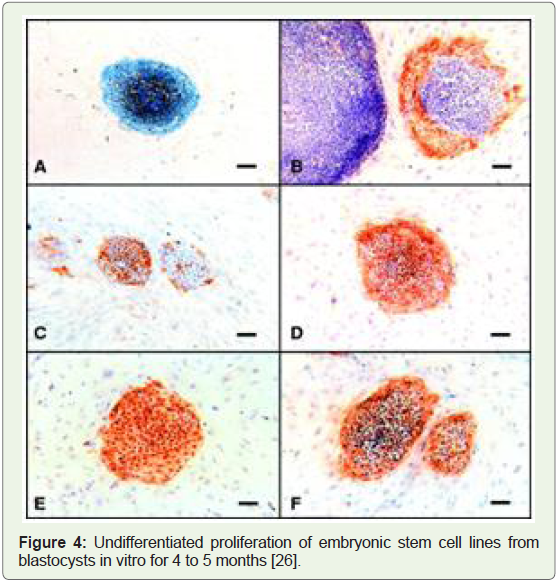
Human embryonic stem cells isolated, and lab organs approved, 1998: H1, H7, H9, H13, and H14 were the very first human embryonic
stem cell lines that were isolated in the year 1998 in a confidential lab
research facilities due to lack of federal funds and lack of widespread
general consensus (Figure 4) [25].
Figure 4: Undifferentiated proliferation of embryonic stem cell lines from
blastocysts in vitro for 4 to 5 months [26].
Somatic cell nuclear transfer cloning of mammals, 1999: The
several kinds of somatic cells employed for these highly successful
and result oriented livestock cloning were: mammary epithelial cells,
ovarian cumulus cells, skin fibroblast cells, various internal organ cells
from liver, testis, skin, ear, macrophages, leukocytes, cumulus and
oviductal cells. Statistical analysis of the data revealed that cumulus
and oviduct epithelial cells were the most suited for creating healthy
and viable cattle [27].
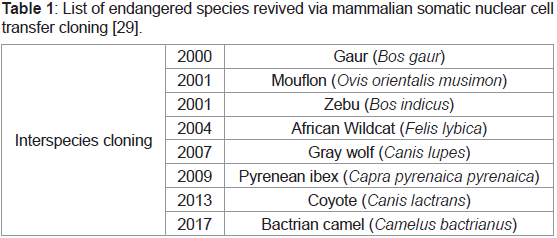
Endangered species cloned via somatic cell nuclear transfer, 2001: One of the unexpected beneficial applications of somatic cell
transfer was revival of endangered species through inter-species
cloning and examining the potential of the same for reproductive
and therapeutic cloning. Examples of such include gaur, mouflon,
zebu, gray wolf, bactrian camel etc (Table 1) [13]. In the same year,
i.e. 2001, the European Union (EU) eased many of the restrictions on
embryonic stem cell research. However, the government funding was
still not approved yet [2,28].
Table 1: List of endangered species revived via mammalian somatic nuclear cell
transfer cloning [29].
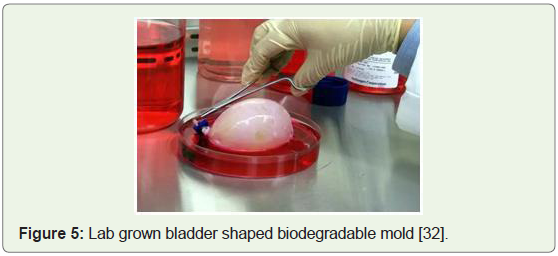
Lab grown bladders for kids & embryonic cells cultured, 2006: The very first human recipients of lab engineered bladders were
reported by Wake Forest researchers in a report that was published
in Lancet Journal together with the long-term success in pediatric and adolescent patients, between the ages of 4 and 19, who received
bladders grown from their own cells (Figure 5). Therefore, avoiding
the risks of rejection and establishing tissue engineering as a viable tool
to solve medical problems of complex magnitude [30]. Furthermore,
research data demonstrated in the same year that embryonic stem
cells can be directly generated from mouse adult cells by inducing few
but specific cell culture transcription factors [31].
Figure 5: Lab grown bladder shaped biodegradable mold [32].
Rhesus monkey embryonic stem cells created, 2007: Derivation
of embryonic stem cells genetically identical to a primate by somatic
cell nuclear transfer was achieved and results represented successful
reprogramming of adult somatic cells into embryonic stem cells. This
proved that therapeutic cloning in primates is practical, and it also
holds enormous potential to cure several degenerative diseases by
nullifying concerns about rejection by the host immune system [33].
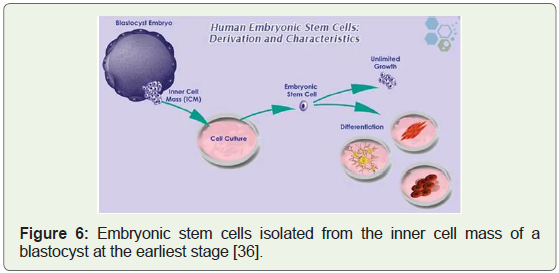
Human embryonic stem cell lines approved, 2009: Federal
funding and grants were first approved for research on human
embryonic stem cells in 2009. After several years of debate and
legal challenges, a new era emerged (Figure 6) with the potential for
breakthrough in health and medicine [34]. 2009 was also the year
when for the first time an extinct goat species of Spain was cloned
from cryopreserved skin biopsy samples that were collected in 1999
(Figure 7) [35].
Figure 6: Embryonic stem cells isolated from the inner cell mass of a
blastocyst at the earliest stage [36].
Figure 7: Extinct Spanish mountain goat species called the bucardo cloned
via cryopreserved skin biopsy sample [37].
Breakthrough cures for spinal cord injury and cornea repair, 2010: Novel biomaterials were designed to develop hydrogel scaffolds
for clinical cure of the most debilitating and complex injuries of the
spinal cord. These scaffolds could stimulate cellular regeneration
and functional recovery and involved the combinatorial approach
of integrating biomaterial scaffolds with cell transplantation and
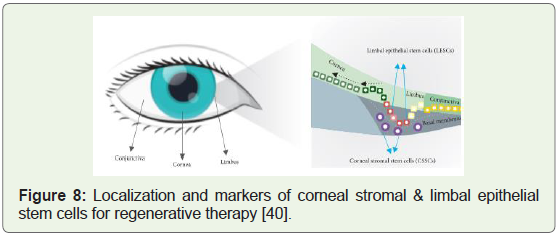
molecule delivery [38]. The unique challenge of corneal tissue injury
was delved deeply after the discovery of the two types of stem cells
that were given the names (Figure 8): CSSC’s & LESC’s; the full
versions being corneal stromal stem cells and limbal epithelial stem
cells respectively; and both types of stem cells were found to have
huge implications to invent clinical cures for Cornea related health
conditions [39].
Figure 8: Localization and markers of corneal stromal & limbal epithelial
stem cells for regenerative therapy [40].
Human stem cell lines created by virtue of therapeutic cloning, 2013: After many years of trying, researchers at Oregon health
sciences university harvested skin cells from a baby with a congenital
health condition. They succeeded in fusing them with donated human
eggs to create human embryos that were genetically identical to the
donor baby and then efficiently derived stem cells from the embryos
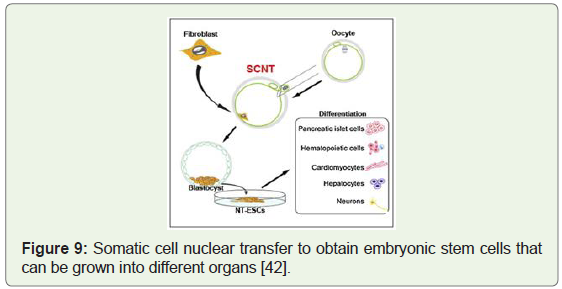
[41]. Image below shows the somatic cell nuclear transfer to obtain
embryonic stem cells with the potential to be grown into pancreas
(Figure 9), heart (Figure 10), liver, brain and red blood cells in vitro.
Figure 9: Somatic cell nuclear transfer to obtain embryonic stem cells that
can be grown into different organs [42].
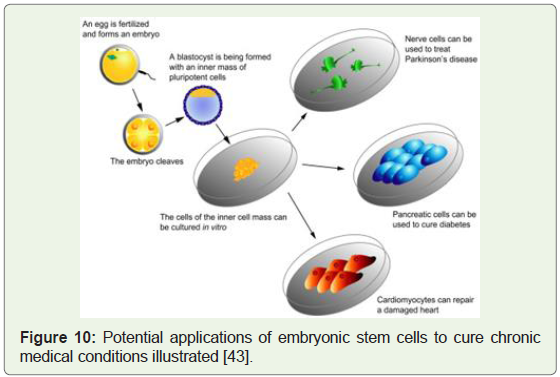
Figure 10: Potential applications of embryonic stem cells to cure chronic
medical conditions illustrated [43].
Patient specific stem cells created by STAP technique, 2014: A
unique and simple cellular reprogramming method was invented
by Obokata et.al. Termed as stimulus-triggered acquisition of
pluripotency (STAP), which eliminated the need for both nuclear
transfer and transcription factors in order to grow stem cell lines
in the lab [44]. The discovery was that a low ph. medium triggers
somatic cells to give rise to STAP cells by reprogramming rather than
selection. This also proved that STAP cells efficiently contribute to
chimeric embryos and off springs. Further derivation of robustly
expandable pluripotent cell lines was also demonstrated [45].
Stem cell therapy to cure severely damaged cornea, 2015: Promises and ambitions to repair severely and deeply hurt corneal
tissue due to physical or chemical burns were fueled when EU
approved a limbal stem cell treatment developed by researchers at
Modena University in Italy. The outer layers of the cornea deteriorate
causing limbal stem cell deficiency [46]. This novel therapy heals by
transplanting corneal sheets which could be grown from the limbal
stem cells derived from the undamaged part of the limbus. This
was found to be good enough to restore the sight to both the eyes
and resulted from over 2 decades of basic, pre-clinical and clinical
research studies [47].
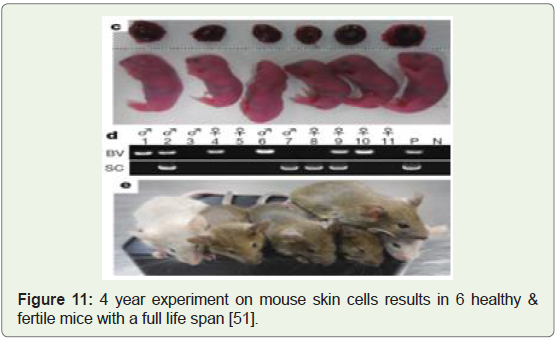
Healthy and fertile mice created from mouse skin cells and surrogacy, 2016: A team of stem cell biologists at Kyushu University
were able to generate healthy mouse pups by maturing skin-cellderived
eggs inside the mouse mother where the maturation took
place in a lab dish [48]. The initial hypothesis was published in 2011
[49], the actual lab experiments took 4 years and resulted in the birth
of 6 baby mice that were fertile, healthy and had normal lifespans,
despite only 1 % of the implanted cells being live births (Figure 11).
The research clearly suggests that women who lack eggs or for men
without sperm, can get replacement cells made from their own skin
therefore extending human fertility by decades, and may also help
preserve endangered animal species and even allow same-sex couples
to have their own genetic children [50].
Figure 11: 4 year experiment on mouse skin cells results in 6 healthy &
fertile mice with a full life span [51].
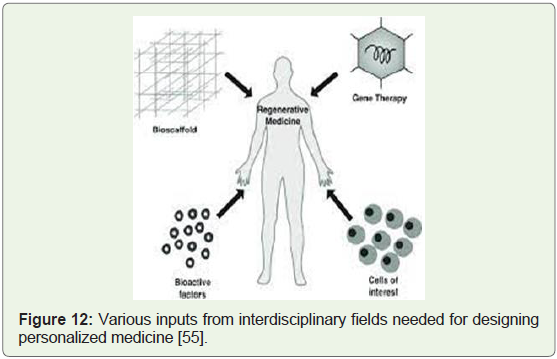
Regenerative Medicine:
Regenerative medicine can be viewed as a promising
interdisciplinary field of research and clinical applications that focus
on the repair, replacement, regeneration of cells, tissues, organs to restore impaired function resulting from causes such as congenital
defects, disease, injury and aging [52]. Clinical procedures aimed
to repair damaged tissue or organs, by clinically engineered tissue
scaffolds, stem cells to replace cells & tissues damaged by aging/
disease via tissue engineering, genetic engineering and molecular
activators; are considered techniques to make new body parts from a
patient’s own cells and tissues (Figure 12). This therefore reduces the
goal of treating depleted organs in the human body to restore normal
function in such a way that there is no need to replace whole organs
[53].
Figure 12: Various inputs from interdisciplinary fields needed for designing
personalized medicine [55].
Regenerative medicine integrates the process of self-healing where
the human body uses its own repair mechanisms, or sometimes with
help foreign biological material to recreate cells, rebuild tissues and
organs (Figure 12). Tissue engineering and regenerative medicine
are considered synonymous as scientists and clinicians utilize this
approach with the hope to focus on cures for complex chronic
diseases [54]. Regenerative medicine encompasses three domains
(i.e., tissue engineering, stem cells and cloning).
Tissue Engineering: Tissue engineering is a field which
progressed from biomaterials development and combines scaffolds,
cells, biologically active molecules into functional tissues. The holistic
goal is to assemble functional constructs that restore, maintain,
improve damaged tissues or whole organs like artificial skin or lab
engineered cartilage that have been approved by the Food and Drug
Administration (FDA) with limited clinical applications [45]. Tissue
engineering’s principles can be characterized into cell transplantation, science of biomaterials or engineering biological substitutes [56].
Tissue engineering’s clinical application strategies can be classified as
acellular scaffolds (i.e., highly dependent on the body’s natural ability
usually prepared via 3d printing artificial scaffolds or by removing
cellular components from tissues) or scaffolds seeded with stem cells
i.e., deployed stand-alone via direct injection or clubbed with carriers
such as hydrogels [56]. Tissue engineering is also considered an
interdisciplinary field that brings together bioengineering, material
science and life sciences to accelerate healing processes through the
assembly of biological substitutes [57]. These biological mockers are
often three dimensional constructs with the function, structure and
mechanics better than the tissue that is to be replaced and such 3-D
constructs demand wise selection of four key materials i.e., scaffold,
growth factors, extracellular matrix and cells [57].
Stem cells in tissue engineering: Native cells can be described as
a variety of primary human cells (e.g., bladder urothelial cells). In
patients with extensive end-stage organ disease, tissue biopsies may
not yield enough normal cells required for growing in vitro cell lines.
In other instances primary autologous human cells cannot expand
or grow from a particular organ (e.g., pancreas). This is where stem
cells come to the rescue as viable alternative sources from which the
needed tissues can be grown in the lab medium and harvested [56].
These procedures involve growing organs in the lab medium through
selection and seeding of the right kind of stem cells that are skillfully
placed onto a scaffold and allowed to mature in a bioreactor, before
surgical implantation with the goal of replacing a malfunctioning
organ.
Stem Cells & Therapeutic Cloning: High quality samples of
autologous cells from the diseased organ of the host are the primary
source for tissue/organ replacement therapy and it is not a viable
option for extensive end-stage organ failure; and hence embryonic
stem cells (ESC) are the alternative from which the desired tissue can
be lab grown through combination of tissue engineering methods
employed over the past few decades [58]. ESC isolated by immunesurgery
of the embryo have demonstrated longevity in culture by
maintaining their undifferentiated state for at least 80 generations
under standard protocols and in addition possess two remarkable
advantages (i.e., the ability to proliferate into an undifferentiated
pluripotent or self-renewal state and to differentiate into many
specialized cell types) [58]. Differentiation into cells of various
types has also been established in skin, neurons, blood, cardiac cells,
cartilage, endothelial cells, urethra, bladder, blood vessels, trachea,
muscle, pancreas etc. & further evidence of their pluripotency was
noticed through cell aggregations both in vivo as well as in vitro lab
cultures [58]. Timely legal, ethical and political barriers to progress in
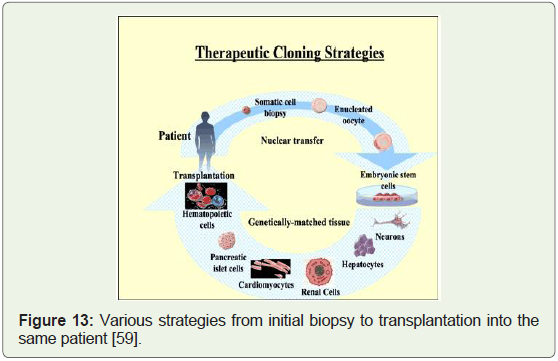
ESC research have led to the search for alternate sources. Therapeutic
cloning can fill this huge void (Figure 13).
Figure 13: Various strategies from initial biopsy to transplantation into the
same patient [59].
Biomaterials in tissue engineering:
Synthetic materials such as Teflon and silicone that were
introduced to replace or to rebuild diseased tissues or parts in the
human body, led to the development of a wide array of devices.
However, the functional aspect of the original tissue was not restored.
This led scientists in cell biology, molecular biology, and biochemistry
to further investigate and as a result novel biomaterials were discovered and designed [56]. These newly developed biomaterials
were able to replicate the biological and mechanical function of
human and animal tissues/organs in a simulated 3-D format in which
cells can attach, grow, form new tissues with appropriate structure and
function and also providing the appropriate cell-adhesion substrate,
growth factors and bioactive factors critical for most mammalian
cell types while allowing delivery of cells with high loading efficiency
under desirable condition in vivo forces so that the predefined 3-D
structure of a specific tissue-engineered organ is attained as a most
desirable clinical as well as public health outcome (58). Classes of
biomaterials found to be most ideally biocompatible for both in vitro
and in vivo engineering of tissues include [58]: naturally derived
materials like collagen and alginate; acellular tissue matrices (bladder
submucosa and small intestinal submucosa); and synthetic polymers
like poly glycolic acid (PGA), poly lactic acid (PLA), poly lactic-coglycolic
acid (PLGA)Cost & Insurance challenges in translating regenerative therapies to clinics:
Unsolved funding issues linger in the domain of innervation
of tissues and organs which is critical for full in vivo functionality
[56]. Several clinical trials involving stem cells and cloning have been
paused due to limited funding [56]. As the cost that the medical health
care system can allow for advanced therapies is limited; insurance
plans are not ready to reimburse for the same [56]. Lowering the
costs of bioengineered products will be addressed and realized as the
technologies gradually advance with time, and the volume of stem cell
clinical impact applications increase [56].Promising areas of tissue engineering:
Researchers at the National Institute of Biomedical Imaging
and Bioengineering (NIBIB) are focused on several areas to rapidly
develop novel clinical therapies by [54]: quality control of stem cells
by regulating their lab culture environment; implanting human livers
in mice to save time and costs of developing new drugs and allow
for monitoring critical drug interactions; engineering mature bone
stem cells to tackle abnormal bone growth; sugar lattices to help
engineered tissue survive isotonic body fluids; solutions like bio gels
and bio adhesives for bum knee; and regenerating new kidneys from
patients own sells to overcome problems of donor shortages and
drastically reduce morbidity associated with immunosuppression in
organ transplants.Applications in health and medicine:
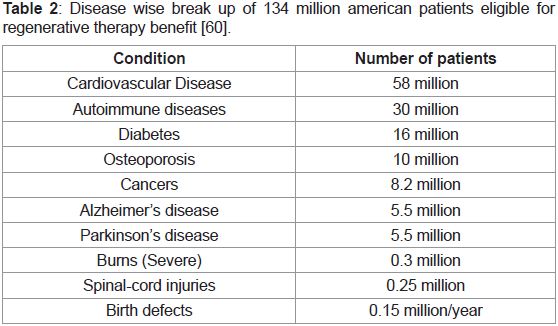
2002 executive summary report prepared by the National research
council’s review committee calculated the number of potential
americans that could benefit from tissue regenerative therapeutic
treatments to be more than 134 million as shown in the break down
based on their individual medical conditions below (Table 2) [60]:
Table 2: Disease wise break up of 134 million american patients eligible for
regenerative therapy benefit [60].
Bioartificial liver (BAL): BAL support system was designed
and developed as a bioreactor cartridge, loaded with detoxifying
hepatocytes and hollow nanofibers acting as an immunoisolation
barrier. This enabled the prevention of direct contact of patient blood
flowing on to the nanofibers with the hepatocytes [61].
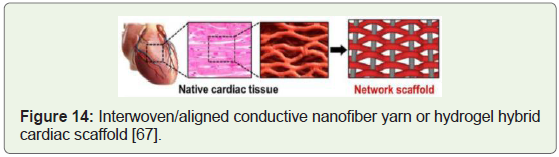
Generation of heart muscle cells: Clinical trials targeting
stimulation of growth of new blood vessels that repopulate heart
tissue instead of complex surgical interventions have proved to be
safe & effective (Figure 14) [61].
Figure 14:Interwoven/aligned conductive nanofiber yarn or hydrogel hybrid
cardiac scaffold [67].
Hematopathology: Research of hematopoietic or blood forming
cells involving both adult and embryonic stem cells gave great insights
into mechanisms of transfusion fluids for critical care medicine,
surgery, organ preservation and improvement of microcirculation in
diabetes, atherosclerosis and sickle cell disease [61].
Neurodegenerative conditions: Clinical trials that harvested
neural stem cells from healthy adult brains were found to be capable
of maintaining stem cell numbers or become progenitor cells with
promise of treating Parkinson’s and Alzheimer’s disease [61].
Spinal cord: Adult stem cells transplanted by scientists into
spinal cord injury patients led to the exciting discovery that human
embryonic stem cells & human blastocyst stem cells can also be
injected into neural stem cells and further probing shed light on
clinical solutions by experimenting with motor neurons & spinal
motor neuron cells [61].
Diabetes: Human embryonic stem cells were grown through
cell cultures and tweaked in vitro to form insulin producing cells that were transplanted directly into the pancreas. This restored the
function of their insulin producing beta cells [61].
Cancer: Bone marrow and umbilical cord stem cells have been
successfully used to treat conditions such as leukemia and lymphoma
by recouping hematopoietic stem cells killed by the cytotoxic
chemotherapy agents within the bone marrow [61].
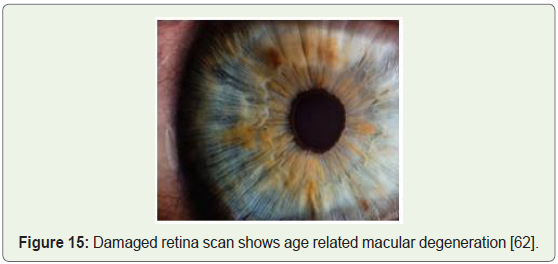
Blindness and vision impairment: Researchers achieved success
via transplanted retinal stem cells into damaged eyes (Figure 15),
again by employing embryonic stem cells. Thin sheets of totipotent
stem cells grown in the lab were transplanted over the damaged retina
stimulating renewed repair and subsequently restoring vision [61].
Figure 15: Damaged retina scan shows age related macular degeneration [62].
Amyotrophic lateral sclerosis (ALS): Clinical benefits of stem
cells were proven in curing rats with ALS like disease by injections
of stem cells into their spinal cords which then passed through many
layers of tissues to the specific sites of injury, regenerating the dead
nerve cells. This restores the patient’s abilities to walk again [61].
Baldness: Researchers predict and expect that research on hair
follicle stem cells may lead to successes in treating baldness through
hair cloning performed by harvesting stem cells from existing follicles
and multiplying the same through in vitro cultures further leading to
implanting the new follicles [61].
Dental cures: Stem cells collected from the patient were worked
on in the lab to grow new tooth buds and surgically implanted into
the gums, giving rise to new healthy teeth by jawbone fusing through
releasing chemical messengers that encouraged growth of new oral
nerves and blood vessels [61].
Graft versus host disease and Crohn’s disease: Novel
breakthrough intravenous therapies developed by stem cells derived
from adult bone marrow of 18- to 30-year-olds, abundant in
mesenchymal stem cells (63), have enabled researchers to successfully
target disorders related to both graft versus host and Crohn’s disease
[61].
Neural and behavioral birth defects: Direct neural stem cell
transplantation into the brains of the offspring was found to bear fruit
in inducing the host brain to produce large numbers of stem cells
which repaired the neuronal and congenital damage [61]. Series of
steps included taking cells from the patient’s own body, turning them
into stem cells, and then transplanting them back into the patient’s
blood [61].
Orthopedics: Clinical case reports in the treatment of orthopedic
conditions have been reported. Centeno et al. Have published MRI evidence of increased cartilage and meniscus volume in individual
human subjects, though it is unclear how the MRI results compare to
clinical response.
Veterinary applications: Research conducted on horses, dogs,
and cats has shown potential to develop stem-cell treatments in
veterinary medicine and may contribute to human medicine cures
for myocardial infarction, stroke, tendon and ligament damage,
osteoarthritis, osteochondrosis and muscular dystrophy [61].
Companion animals were found to be superior models than typical
mouse models and armed with relevant Veterinary research since
1998; regenerative treatment models sprouted involving mesenchymal
stem cells harvested primarily from adipose tissue or bone marrow in
order to treat animals with injuries or defects affecting bone, cartilage,
ligaments and tendons [61].
Mechanisms of action: Scientific evidence has supported
encouraging facts that stem cells improve healing through below
observed mechanisms: anti-inflammatory effect; homing to injured
tissues; recruiting tissue growth factors; supporting tissue remodeling
over scar formation; inhibiting apoptosis and differentiation into
bone, cartilage, tendon, ligament, muscle, fat and other tissues [61].
Limitations & ethical considerations
There are some limitations of the research work on tissue
engineering and regeneration that have been done to date.
1) Data cited in research studies are mostly not reproducible [64].
2) Designing appropriate capillary networks to allow gas
exchange, provide nutrition, and remove metabolic waste
from the implants [64].
3) Different cell types require unique culture mediums which
makes it quite challenging to design a multilayered organ
system scaffold [64].
4) Designing and creating a scaffold capable of supporting various
cell types [64].
5) In order to maximize ESCs in tissue growth & maintenance
of the pluripotent state; expression sequence, dosing, and
duration for growth factors have to be pre-defined accurately
[64].
The ethical considerations in stem cell research include:
1) Adult stem cells (little controversial)
2) ESC’s from discarded embryos produced in vitro (most
controversial)
3) ESC’s obtained through therapeutic cloning (extent of
controversy related to type of research)
4) ASC’s (adult stem cells) may as well avoid the ethical problem
of ESC’s but not a great deal of scientific data is published
regarding their replication and differentiation patterns [64]
5) ESC’s have clear cut technical advantages over ASC’s as they
can be generated in abundant quantities in the lab and in
undifferentiated state for many generations [64]
6) Researchers face great adversity in creating ideal lab conditions
for adult hematopoietic stem cells that can proliferate without
becoming specialized; thereby limiting the avenues to explore
ASC’s to generate specialized cells in abundance needed for
transplantation [64]
7) Despite impressive progress in the field of tissue engineering,
further work toward organ and tissue replacement is crucial
and optimal cell sources, 3-D designs, and microfabrication
technology are still being investigated [57]
8) The search for ideal mammalian multipotent or pluripotent
stem cells in tissue engineering has been emerging rapidly
and also associated with controversies [57]
Future with stem cell therapy:
Human ESC have proved their ability to differentiate into somatic
cell types that can grow the entire human body and potential benefits
to health and medicine are very wide ranging, from generating
neurons for Parkinson’s patients to learning about the underlying
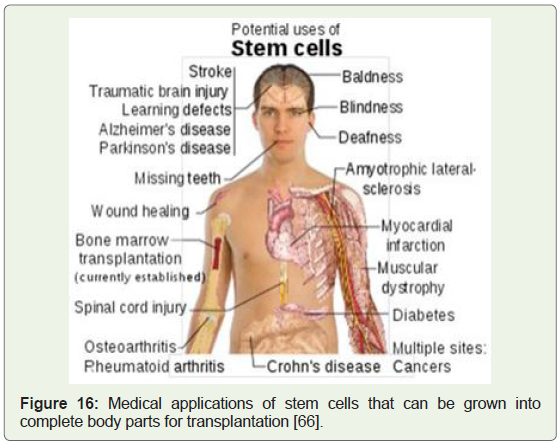
molecular biology and biochemistry of tumor development (Figure 16) [65]. A highly cited 2002 report by the National Academies of
Science estimated that the potential patient population in the US
eligible for stem cell-based therapies are more than 100 million [60].
Figure 16: Medical applications of stem cells that can be grown into
complete body parts for transplantation [66].
Conclusion
After reviewing progress that has been made in the several
domains of regenerative medicine, scientists are constantly
improving and expanding these cost effective therapies. The shortage
of donors increasing along with increasing patient populations
each year, the boomer generation’s needs and demands for surgical
implants and increased life expectancy, regenerative medicine will
become more appealing as a therapeutic solution in the future. The
acceptance of regenerative medicine as a therapeutic option could
have both public health and economic implications in the healthcare
system. Regenerative medicine has the potential to improve health
outcome and quality of lives; reduce healthcare costs; reduce or
eliminate the risk of immune system rejection; eliminate the search for a matching donor or the need of a donor itself completely; by pass
immunosuppression medicines and related adverse events; shorten
the rehabilitation periods and expenses; ease the burden of inpatient
and outpatient visits in super busy hospital and clinics; design/
engineer and print 3-D tissues and organs in shorter time frames;
and shorten the phases of clinical trials. As more research work is
done, regenerative medicine will be more recognized and would be
considered as a treatment option.
References
28. Birmingham K (2003) Europe fragmented over embryonic stem cell research. J Clin Invest 112: 458.