Research Article
Heat Shock Induced Triple Helix DNA and it’s Refolding to Duplex by HSP70 in Yeast
Pasha SM1, Musfera S2, Hari Narayana Kola1,3, Rao LV1 and Pasha C1*
1Department of Microbiology, Nizam College, Osmania University, Hyderabad, India
2Department of Microbiology, Shantinikethan Women’s College, Osmania University, Hyderabad, India
3Department of Biotechnology, Vignan’s Foundation For Science Technology & Research, Deemed to be University, Vadlamudi,
Guntur District, Andhra Pradesh, India
*Corresponding author: Pasha C, Department of Microbiology, Nizam College, Osmania University, Hyderabad, India; E-mail:
cpasha21@gmail.com
Copyright: © 2023 Pasha SM, et al. This is an open access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 10/12/2022; Accepted: 11/01/2023; Published: 16/01/2023
Abstract
Heat can damage and inhibit the synthesis and repair of damage to DNA, proteins, RNA. Heat shock increases DNA damage by crosslinking and
breakages. Crosslinking creates the multi strand DNA, which down regulates many genes. Triple helix is a major form of multi strand DNA reported to exist.
Using poly purine templates for BLAST, number of possible triple helical structures were predicted. Frame shift assays using denaturing PAGE, UV spectra,
melting complexes, DMSO foot printing and RNAse T treatment were analyzed for triple helix formation. Heat shock caused accumulation of triple helix DNA
in yeast. Heat shock proteins were reported and found to bind the triple helical DNA. Double stranded DNA structure was retrieved from triple helix with the
help of HSP 70. This study confirms the possible role of HSP 70 for refolding of misfolded DNA like that of proteins.
Keywords
Triple helix DNA; Heat shock; HSP; Thermotolerance; Yeast
Introduction
Research over years has yielded clear evidence that DNA is the
molecule responsible for the inheritance of traits from one generation
to the next. DNA is usually double stranded; two strands of
nucleotides are attached to one another via Hydrogen bonds between
bases. Many of these sequences adopt the orthodox right-handed B
form, probably for the majority of the time, with Watson-Crick A:T
and G:C bp. However, at least 10 non-B conformations are formed,
perhaps transiently, at specific sequence motifs as a function of
negative supercoil density, generated in part by transcription, protein
binding, and other factors.
Multistranded DNA structures have attracted a great attention in
recent years, as they possibly play a substantial role in chromosomal
DNA organization and regulating gene expression [1-4]. Triple
helix DNA was first discovered in a complex of poly (A) and poly (U) by Felsenfeld, Davis and Rich in 1957 [5]. Arnott and coworkers
established the triple helix DNA model by X-ray diffraction analyses
in 1974 and ever since researchers are resolving DNA triple
helix structures [6,7]. Studies of triple helix DNA have been paid
much more attention because of its importance as a tool for DNA
sequencing, gene control and therapeutic applications [1]. The triple
helix provides means to design powerful artificial endonuclease when
the third strand is coupled with a cleaving agent. The triple helix
forming activity also holds strong promise in the areas of genome
mapping. Triple helix-DNA provide potential tools for altering
gene function by either repressing transcription, inhibiting DNA
replication or inducing site-specific mutagenesis and recombination
[8,9]. It was found that the population of triple helix-forming target
sequences is much more abundant, especially in promoter zones,
which suggests a tremendous potentiality for triple helix strategy in
the control of gene expression [10]. Studies from human genetics and from model organisms indicate that the Triple DNA itself plays
a major role in its own mutability [11]. The intermolecular triple
helix has been shown to inhibit gene expression in vivo, including a
case demonstrating the inhibition of HIV-1 transcription in infected
human cells. Medically related issues have been vigorous motivators
of triple-stranded research. At least 20 hereditary neurological
diseases are caused by the expansion of simple triple helix sequences
in either coding or non-coding regions [12]. It has been previously
demonstrated that triple helix formation at a promoter can block
the binding of various transcription factors thereby inhibiting
transcription. Another mechanism may be to prevent transcriptional
elongation. Intermolecular DNA triple helixes have pronounced
sequence recognition properties and have been used successfully to
decrease transcription from specific genes [13].
Cells and tissues are challenged constantly by exposure to extreme
conditions that cause acute and chronic stress. Consequently, survival
has necessitated the evolution of stress response networks to detect,
monitor, and respond to environmental changes [14]. Prolonged
exposure to stress interferes with efficient operations of the cell, with
negative consequences on the biochemical properties of proteins,
Nucleic acids that, under ideal conditions, exist in thermodynamically
stable states. In vitro studies revealed the formation of triple helix
DNA by heat shock [15]. The ethidium bromide fluorescence assay
detects DNA interstrand crosslinks following heat denaturation of
DNA [16]. Heat shock proteins role in refolding of thermal stress
induced misfolded proteins is well established, where as few reports
stating the similar role of HSPs on Nucleic acid aggregates [17].
There are now several areas of research that target the problem of
triple helix stability. One possible strategy, with which we concerned,
is to use compounds that bind spherically to triple helix (not duplex)
DNA, thereby facilitating the formation of double helix structures. In
present study we were attempted to found heat induced triple helix
formation and its reversal by HSP70 treatment.
Materials & Methods
Culture Conditions:
Microorganism: Saccharomyces cerevisiae - VS3 was isolated from
soil samples collected from hot regions near the Kothagudem Thermal
Power Plant located in Khammam District, TS, India. The organism
was isolated, mutated by UV and identified as Saccharomyces
cerevisiae -VS3 strain in our lab [18]. It was maintained on Yeast
extract Peptone Dextrose agar medium (YEPD) (1% Yeast Extract,
2% Peptone, 2% Glucose, 2% Agar-Agar, pH 6.0).
Chemicals & enzymes:
Human HSP 70, Yeast Lyticase, RNAse was obtained from Sigma
Aldrich.Remaining chemicals and media reagents are obtained from
HIMEDIA chemicals.
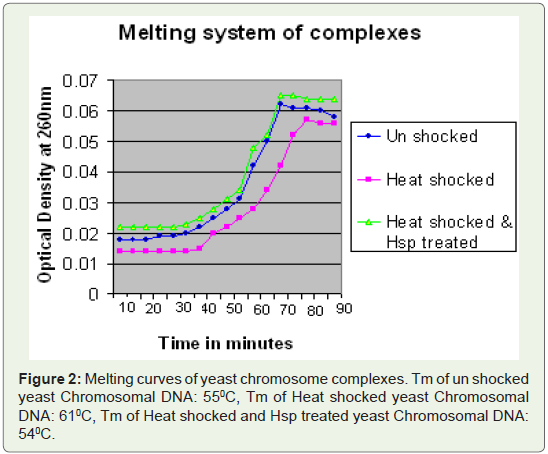
Prediction of Triple helix DNA in Yeast:
Query sequence GAGAAAAATGAA with poly purines,
reported to form triple helix DNA was used to BLAST (Search for
short, nearly exact matches) against yeast genome database available with NCBI Gene bank [19]. Results obtained (top 10) were checked
for their function analysis from NCBI database [20].Confirmation of Triple helix DNA:
Yeast Chromosome preparation and Heat shock:
Thermotolorant yeast Saccharomyces cerevisiae VS3 strain was
grown in YEPD broth for 48 hours at 30°C and 150 rpm. Yeast cells
washed with 0.5M PBS and were suspended in protoplast solution
(0.5M PBS, 6M sorbitol, 2mM Beta Mercapto ethanol , 100U lyticase
and 1μl of Rnase (50 ng/μl)). The mixture was incubated at 30oC and
50 rpm for 2 hours. Samples were collected at an interval of 10 minutes
up to 2 hours and checked for protoplast conversion by methylene
blue staining, spherical shape of protoplasts and hypotonic rupture of
protoplasts. After 90% cells were converted to protoplasts, heat shock
was given at 50 and 60°C for 45 minutes.Nondenaturing PAGE assay for triple helix formation:
Rnase and Lyticase treated yeast suspension was centrifuged at
22000 rpm and supernatant containing enzymes were removed.
The pellet was used for phenol extraction of DNA. Then 5ml of the
mixture was combined with 1 ml of 6X aqueous glycerol containing
xylenecyanole and Bromphenol Blue. The products were resolved
in 20×20 cm slabs of 4% gel (acrylamide/bisacrylamide 19:1 with
Ethidium Bromide) in TAE at 24°C for 16-18 h at 60 mA, 110 V.
Bands were visualized by Transilluminator in gel documentation.Dimethyl Sulfate (DMS) Footprinting:
Heat shocked chromosome preparations (250μl) was purified
by phenol extraction and treated with 10 μl of 1:40 dilution of DMS
in dimethyl sulfoxide for 3 min at 0°C. Reactions were stopped by
DNA precipitation with ethanol. For both DNA cleavage of DMS treated
substrates and removal of crosslink, pellets were dried and
resuspended in 100 μl of 0.1 M NaOH and heated to 90°C for 1 h.
After an additional precipitation, samples were resuspended in 5 μl of
loading buffer and loaded on an 8 M urea/4% poly acrylamide gel and
used for electrophoresis under denaturing conditions.HSP Treatment
After heat shock at 50°C protoplasts were treated with pure HSP70
(Sigma) and incubated at 37°C for 1 hour. Samples were extracted with
phenol/chloroform, and products were precipitated with ethanol.
Theses samples were used for electrophoresis and estimation of Tm.
Thermal stability:
Thermal stability of complexes was assessed by monitoring the
UV absorption of the solution in Spectronic spectrophotometer while
the temperature of DNA sample was steadily raised at 1°C/min from
25 to 90°C. The concentration of DNA was 100 μg/10μl in 10mM Tris
HCL buffer.RNase Hydrolysis:
Heat shocked and DMS printed chromosomal DNA (2ml) were
separately treated with 5 μl of RNase solution (50 ng/μl) in a final
volume of 50 μl at 37°C for 1 h. Reaction mixtures were extracted with
phenol/chloroform, and products were precipitated with ethanol.Results & Discussion
The existence of triple helix sequences has been studied by using
bioinformatics tools [10], hence we also approached bioinformatics
mode of predicting triples sequences in yeast genome.
In BLAST analysis, many of the genes (80%) forming triple helix
DNA were not yet characterized. Remaining are expressed but down
regulated after the heat shock [21].
Prediction of triple helix-forming sequences in Yeast (Table 1).
Query sequence GAGAAAAATGAA, Blasted against S.cerevisiae
database.
Confirmation of Triple helix DNA in heat shocked yeast:
Microscopic examination showed spherical Protoplasts. At 25th
minute, 80% protoplast formation was noted. When these protoplasts
were diluted in distilled water, decrease in protoplast count was noted
due to hypotonic rupture. Protoplasts were stained by methylene blue
and observed for loss of membrane bound oxidases (blue stained).
Protoplasts were also stained by using geimsa stain for chromosomes.
Chromosomal DNA isolated from protoplasts showed 16 separate
bands indicating each chromosome as a separate band. Protoplasts
(>80%) after heat shock were found to aggregate the chromosomal
DNA. After heat shock, number of bands was decreased with increase
in shock temperature, indicating the formation of complexes.Dimethyl sulfate (DMS) methylates G residues, creating an adduct
that can be broken chemically. Triple helix complexes are formed
and treated with DMS and then the reaction is stopped with ethanol.
Subsequent treatment with hot alkali breaks the DNA backbone at
methylated sites [22]. Triple helix DNA complexes were found to be
protected from DMS mediated DNA cleavage due to hindered G in
major rove [23]. Heat shocked (500C) and DMS foot printed yeast
showed almost similar aggregates like heat-shocked yeast. Rnase
T cleaves single strand RNA/DNA [24]. Rnase T treatment to heat
shocked yeast DNA yielded many bands confirming triple helical
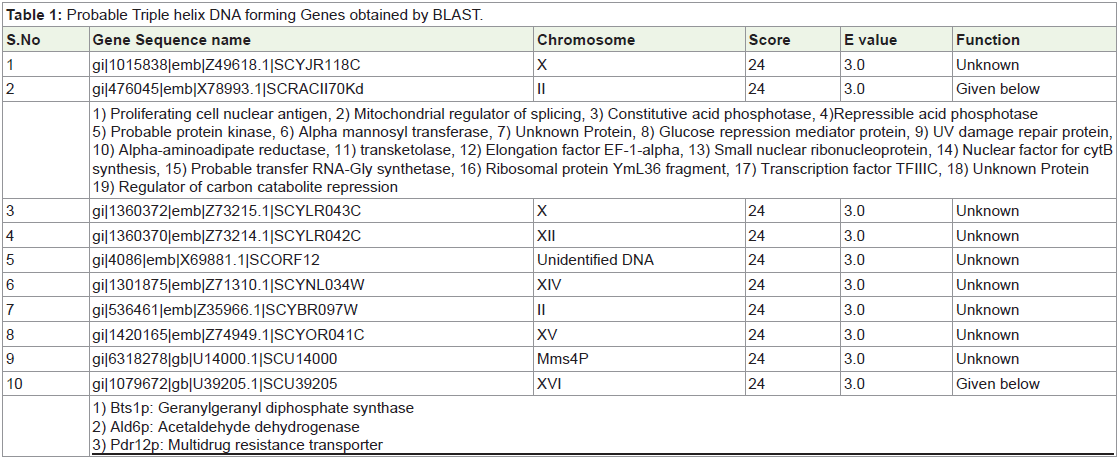
DNA formation by heat shock (Figure 1).
Figure 1: 1) Un shocked Yeast chromosomes; 2) Heat shocked Yeast
chromosomes (at 500C), 3) Heat shocked Yeast chromosomes (at 600C),
4) Heat shocked and RNAse treated Yeast Chromosomes, 5) Heat shocked
and DMS treated Yeast chromosomes, 6) Heat shocked and Hsp treated
Yeast chromosomes, 7) Un shocked Yeast chromosomes.
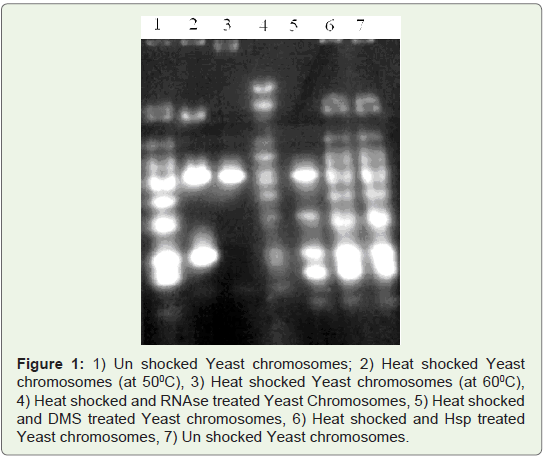
Melting Complexes:
Melting temperature of un shocked yeast DNA was 550C, whereas
in heat shocked yeast DNA, it was 610C. Increased Tm (melting
temperature) of heat shocked yeast Genomic DNA also confirms the
formation of triple helix DNA. Triple helix binding proteins were
detected in human and yeast for their role in making linear duplexes
enabling proper chromosome segregation [25,26]. The molecular
chaperones are housekeeping molecules that assist in the folding and
prevention of the aggregation of proteins and nucleic acids, as well as
participating in the elimination of ubiquitinated molecules [17]. Heat
shocked DNA was treated with Heat shock protein (HSP 70) for 20
minutes. After Hsp, treatment DNA was phenol extracted and used
for detection of melting temperatures. Tm (melting temperature) of
Heat shocked and Hsp treated DNA is similar to un shocked yeast.
Heat shocked and HSP treated Genomic DNA was found to have the
Tm 540C. Heat shocked yeast chromosomes were reversed by Hsp 70
treatment (Figure 2).
Figure 2: Melting curves of yeast chromosome complexes. Tm of un shocked
yeast Chromosomal DNA: 550C, Tm of Heat shocked yeast Chromosomal
DNA: 610C, Tm of Heat shocked and Hsp treated yeast Chromosomal DNA:
540C.
After heat shock, the formation of Triple helix DNA confirms
invitro reports (19). These triple helix structures may be responsible
for blockage of genes after heat shock. Triple helix DNA was converted
to double helix by the treatment of HSP 70. HSP70 can be used to
convert inactive multi strand structures of DNA into the active double
helix and subsequent invitro gene expression to characterize the gene.
Triple helix DNA was known to create the barrier for DNA
polymerase [1]. Down regulation of several genes after heat shock
can be correlated with triple helix formation, over expression of genes
can be explained by their activation by HSPs or selection of different
polymerase or its component like Bacillus during sporulation [27].
Saccharomyces cerevisiae - VS3 produces all major types of HSPs
along with HSP70 after heat stress. HSP70 along with others HSPs
converting triple helix DNA structures to duplexes to retain activity
of most of genes and subsequently conferring in thermotolerance.