Research Article
Multifunctional Silica Nanoparticles for Pancreatic Cancer Specific Drug Delivery and Bioimaging
Preeti Nigam*and Dhiman Sarkar
Corresponding author: Dr. Preeti Nigam, Combichem Bioresource Center, National Chemical Laboratory, Pune-India, Ph.No.: +91-20-25902447, E-mail: ph.joshi@ncl.res.in
Citation: Nigam P, Sarkar D. Multifunctional Silica Nanoparticles for Pancreatic Cancer Specific Drug Delivery and Bioimaging. J Chem Applied Biochem. 2015;2(1): 110.
Copyright © 2015 Preeti Nigam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Submission: 20/04/2015; Accepted: 28/05/2015; Published: 01/06/2015
Abstract
Mesoporous silica nanoparticles (MSN) have attracted increasing interest as drug carriers due to their biocompatibility and robustness. In this analysis we explored the potential of MSN as nanotheranostic platform for pancreatic cancer specific drug delivery and bioimaging. Pancreatic cancer is the most fatal cancer and due to its high mortality and low prognosis it is imperative to develop new methods for early diagnosis and therapy of this cancer. Though traditionally organic dyes, quantum dots and fluorescent agents are used for bioimaging but here we have utilized graphene quantum dots (GQDs) for bioimaging due to their superior biocompatibility and fluorescence properties. MSN were loaded with gemcitabine; the most preferred drug for pancreatic cancer. MSN and GQDs were characterized by SEM, TEM, FT-IR etc and the efficacy of nanoformulation was determined by MTT assay.
Keywords: Pancreatic cancer; Mesoporous silica nanoparticles; Nanotherapy; Drug Delivery; Graphene Quantum dots
Introduction
Nanotechnology based solutions are opening new horizons for solving medical problems and the major advantage associated with nano based therapy is enhanced bioavailability of drugs with reduced side effect. Nanotherapy has the potential to provide more effective and patient friendly treatment regime than the traditional therapy options by reducing drug dosage, targeted delivery of the pay load to a specific site of action with sustain release properties. The delivery of anticancer therapeutics into cancer cells by employing nanoparticles carriers has made significant progress in recent years. Till now many reports are there based on nano drug delivery systems and many reviews have extensively covered this topic [1-3]. In spite of vast research in the area of nano-devices and nano-medicine during the last decade still very few nanoformulations have been commercialized but FDA approval of Serum Albumin nanoparticles-Paclitaxel combination by the trade name ‘Abxrane’ has opened a new gateway for nanocarriers. Several different nano-devices have been suggested to provide a benefit to the current drug delivery scenario and have been applied to prolong the circulation time and bioavailability of drug molecules. Mesoporous silica nanoparticles MSNs) are very promising candidates due to their high internal surface, tunable pore size and volume colloidal stability and ease of functionalization. In recent years great endeavors have been made in controlling of their size, morphology, surface properties and functionalization of MSNs to develop their applications in the fields of biomedicine, biosensing, catalysis, environmental protection and bone repair and scaffold engineering, etc [4-6] Apart from organic nano vehicles, inorganic MSN can also be used to overcome common issues of conventional systemic drug supply such as poor solubility, limited stability, rapid metabolization and excretion of the drug, undesired side effects, and the lack of selectivity towards specific cells types. The encapsulation of therapeutics within MSN that selectively target certain cell types or tissues represents a promising strategy to address these problems. Mesoporous silica materials were discovered in 1992 by the Mobile Oil Corporation and firstly MSNs were used as nanocarriers in 2001 for transporting therapeutics [7]. Till now many efforts have been made in recent years to create Multifunctional stimuli-responsive nanocarrier systems consisting of MSNs based on their excellent material properties [8-10]. To design an efficient cancer treatment, target delivery is vital to overcome the harmful effects of traditional chemotherapy while increasing the drug concentration at the site of action. In general, nanoparticles have a natural tendency to accumulate at cancer cells due to the complex structure of cells as compare to healthy cells. This referential accumulation is caused by Enhanced Permeability and Retention Effect (EPR). But to further increase the efficiency of payload delivery, nanoparticles are tagged with different targeting moieties i.e. folic acid (FA), lactose, mannose and trasferrin etc [11-14]. This improved approach along with EPR effect is better than the earlier nanoparticles mediated drug delivery methodologies. In this study we have designed a multifunctional MSN based nanoplatform for pancreatic cancer specific drug delivery and bioimaging. Pancreatic cancer or pancreatic ductal denocarcinoma is the fourth leading cause of cancer related deaths worldwide with worst mortality rate [15-17]. Late diagnosis in generally advanced stages of cancer, dense stromal vasculature and resistance to chemotherapeutic agents, including gemcitabine (GEM), which is frequently used as firstline therapy, are the major causes of its high portality rate. The 5 year survival rate of pancreatic cancer has remained unchanged in spite of significant advancement in detection techniques and therapy strategies. That makes it imperative to design new strategies for pancreatic cancer specific drug delivery. Earlier few reports of nanoparticles based drug delivery approaches for pancreatic cancer are there [18,19] but still a lot of scope is there to improvise the treatment modes with better payload delivery, stable formulations and higher drug shell life in-vivo with enhanced metabolic rates. In this work, we have designed a multifunctional MSN based nanoplatform functionalized with hyaluronic acid (HA) and grapheme quantum dots specific for pancreatic cancer therapy and diagnosis. It is a well proven fact that CD44, a cancer stem cell marker present on pancreatic cancer cells is one of the prime factors responsible for drug resistance and reoccurrence of this deadly cancer [20]. In this regard, nanoformulations specifically targeting CD44 positive cells can overcome the drug resistivity of pancreatic cancer. Hyaluronic acid is a biodegradable, biocompatible and non-immunogenic glycosaminoglycan having affinity towards CD44 cells and researchers have utilized HA to design targeted delivery systems for pancreatic cancer as well as triple negative breast cancer having HA targeting moiety [21,22]. To develop a complete nanotheranostic system, bioimaging module is also an integral part of the nanoformulation. Here we have utilized graphene quantum dots for imaging and analyzing the nanoparticles internalization. Graphene quantum dots are zero dimension graphene moieties having unique optical and physico-chemical properties. Due to their stable fluorescence and cost effective synthesis approaches with excellent biocompatibility GQDs are gaining much attention for bioimaging and drug delivery as compare to semiconductor quantum dots and organic dyes. To the best of our knowledge, this is the first combination of MSN based nanotheranostic system coupled with HA and GQDs. Gemcitabine; the most preferred drug for pancreatic cancer was loaded on MSN to determine the anticancer efficacy of the nanoformulation.
Materials and Methods
Chemicals
Tetraethylorthosilicate (TEOS), Cetyl trimethyl ammonium bromide (CTAB), (3-aminopropyl) triethoxy silane APTES), N-hydroxysuccinimide (NHS), N-(3-dimethyl aminopropyl)-Nethyl carbodiimide hydrochloride (EDC), sodium hyaluronate (HA), Graphene. All the chemicals were obtained from Sigma and used as received. Double distilled or MiliQ water was used to prepare all the solutions.
Synthesis of MSN
In a typical synthesis, 0.5 g CTAB was dissolved in 240 ml water under stirring at room temperature followed by he ddition of 1.75 ml NaOH (2M, pH=12.4). The temperature of the solution was raised and kept at 800C to make the solution homogenous. To this solution 1.5 ml TEOS was added. The solution was centrifuged and the resulting pellet has collected. The templates i.e. CTAB were removed by adding ethanol-hydrochloric acid (HCl) to the pellet and kept for 12-15 h. The resultant product was again washed with ethanol (thrice), centrifuged and the pellets were dried in a hot air oven.
HA conjugated MSNs preparation
Graphene quantum dots synthesis and conjugation of GQDs with MSNs
Graphene quantum dots were synthesizes via hydrothermal procedure as earlier described by us [18]. In brief, Graphene Oxide (GO) was synthesized by modified Hummers process [23] and subsequently, Chemical reduction of GO solution was achieved using Lawsone (2-hydroxy 1,4 naphthoquinone) as reducing agent under a hydrothermal environment. Typically 100ml of above GO solution was sonicated for half an hour and then 2.87 mM of Lawsone was added with magnetic stirring for 15 min. The solution was then transferred to oil bath and heated at 200 degree centigrade for 4h. The reduced graphene were collected after centrifugation followed by washing with deionised water several times and then purified using dialysis membrane for 24h to reduce size into quantum dots. Not all amino groups in NH2-MSNs reacted with HA, thus the remaining free NH2 moieties were utilized for labelling GQDs via EDC-NHS coupling as described above.
Characterization
Nanoparticles size and morphology
SEM and TEM (The Netherlands), were performed to identify the morphological details of MSN and functionalized MSN and GQDs. For SEM a diluted sample of nanoparticles was drop cast on silicon wafers and dried properly for observation while for TEM, a diluted sample of GQDs and MSN was drop cast on copper grids.
UV-Vis Spectroscopy
UV-Vis spectroscopy (Cary50 Bio, Varian, The Netherlands), was done to obtain the absorption spectra of NPs in the range of 200-800 nm. Samples were measured in I cm path length in a quartz cuvette at 250°C. All the experiments were performed in triplicate.
Fourier Transformed Infrared Spectroscopy (FT-IR)
FT-IR spectra of lyophilized samples of MSN and HA- MSN andGQDs was taken by FT-IR spectrometer (Nicolet iS5, Thermo scientific, USA). The samples were measured within the range of 500- 4000 cm-1.
Photoluminescence Spectra of GQDs
Photo Luminescence (LS 55, Perkin Elmer, USA) spectroscopy was performed to observe the variation in fluorescence properties of GQDs before and after functionalization between 250-700 nm. The emission spectrum was taken at different wavelength from 330-480 nm excitation wavelengths.
Drug encapsulation and drug release profile
Gemcitabine encapsulation and release profile was established via UV-Vis spectroscopy. The gemcitabine loaded HA conjugated MSNs were centrifuged at 12,500 rpm for 20 min. The collected pellet was washed twice and resuspended in deionized water. After freeze drying the powdered sample was stored at 40°C for further use. The supernatant having unencapsulated drug was collected separately and absorbance was measured at 275 nm. Based on the standard curve of gem, free drug concentration was calculated. Encapsulation efficiency (EE %) was calculated from the following formula:
EE (%) = [(CTD – CFD) / CTD] x100 Equation-1
Where, CTD is concentration of total drug and CFD is concentration of drug in supernatant. In vitro drug release profile of gem from nanoparticles was carried out in PBS buffer (pH 7.4). 1.0 mg nanoparticles were dispersed in 25 ml of PBS and were placed in shaker-incubator at 37°C 100 rpm. To estimate the release % of gem, at predefined time intervals of 2 hrs, 3 ml of supernatant from the sample was withdrawn and absorbance was recorded at 373 nm. After sample removal, the suspension was refilled with 3 ml fresh PBS.
Gem % release = CDS/ CTd X100 Equation-2
Where, CDS is the concentration of gem released in the suspension and CTd is the total amount of gem encapsulated in the nanoparticles.
In vitro cytotoxicity and Imaging analysis
MTT (3-[4, 5-dimethylthiazol-2-yl]-3, 5-diphenyltetrazolium bromide) assay was performed to analyze the efficacy of our nanoformulation. Panc-1 (pancreatic cancer cell line) was incubated in DMEM media with 10% FBS and 50 μL gentacyin at 370 C in 5 % CO2 environment. Cells were seeded in 96 well plates for 24 hrs and MSN, MSN-HA, Gemcitabine, MSN-Gem-HA and GQDs were added to cells and further incubated for 24 hrs into the same environment. After second incubation, MTT (10 μl, 10mg/ml) was added and kept for 4 hrs. Subsequently, culture media was removed and DMSO (150 μl) was added and after 15 minutes incubation with shaking, optical density (OD) was taken at 570 nm. All the experiments were performed in triplicate to avoid experimental errors. For imaging, GQDs-MSN and HA-GQDs-MSN were injected into the Panc-1 (Pancreatic cancer cell line) and incubated for 12 h. Panc-1 cell lines were maintained in DMEM media supplemented with 10% fetal bovine serum in 5% CO2 atmosphere at 370 °C. Cellular uptake of nanoformulation was visualized by HCS system (Array Scan, Thermo Scientific, USA).
Results and Discussion
Physico-chemical characterization of MSN, GQDs
This analysis presents a new targeted delivery system for pancreatic cancer; the most fatal among all the cancers. We have loaded gemcitabine on multifunctional mesoporous silica nanoparticles. Gemcitabine is the most preferred drug for pancreatic cancer treatment but its rapid metabolism and low bioavailability are the major factors masking its therapeutic benefits. In this regard, drug encapsulated nanocarriers can provide a better alternative in terms of sustained drug release profile and better bioavailability. Though different polymeric delivery systems have previously been reported [24-26] but here we utilized silica nanoparticles for targeted delivery of gemcitabine and the therapeutic efficacy of the nanoformulation was determined in-vitro against Panc-1 cancer cells. Figure 1 is an illustration of nanoformulation preparation and mode of action.
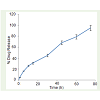
MSNs were synthesized via CTAB template- hydrolysis method and were further functionalized with HA and GQDs by generating NH2 groups on the surface of MSN as described above. Ease of functionalization is another important property that makes MSNs one of the most explored materials for drug and gene delivery. Morphological details of MSNs were obtained from SEM and TEM. The electron microscopy revealed that the as synthesized MSNs were 63-80 nm spheres with average pore size of 1-2 nm as shown in (Figuer 2). Nanospheres display hemotherapeutic advantages of biodegradability and enhanced permeability and retention effect (EPR) for cancer treatment [27]. Thus, these are the most applied form of drug delivery vector. Earlier studies have also shown that phagocytosis of tumor cells depends on size of nanoparticles and nanoparticles with 100-200 nm diameters exhibit preferential accumulation on tumor cells than healthy cells [28]. In previous reports folic acid conjugated MSNs have been synthesized and applied for targeted drug delivery [29,30] but folic acid receptor is present on healthy cells as well, that limits the utility of FA conjugated systems as an efficient targeted delivery systems. So it is crucial to depend on other cell surface receptors more specific to cancerous cells only, for active receptor-ligand targeting strategies. In the present study NH2 groups were generated on MSNs surface to functionalize it with hyaluronic acid. HA is a naturally occurring polysaccharide and is the prime endogenous ligand for CD44; the cell surface receptor expressed on a variety of cancer cells specifically and also plays an important role in cancer development, metastasis and in the interaction between cancer cells and extracellular matrix. Pancreatic cancer cells are rich in CD44 receptors which are responsible for the chemotherapy resistance and recurrence of cancer after treatment [20]. In this regard, a delivery system based on HA conjugated nanoparticles can be very valuable against drug resistant pancreatic cancer and may improve the life expectancy in this fatal disease. The XRD spectrum of MSNs showed three well resolved diffraction peaks that can be assigned to the 10, 11 and 20 reflections of a well ordered two dimensional (2D) hexagonal mesostructure as illustrated in Figure 3. These results were in accordance with the earlier reports [30,31]. MSNs were further modified with APTES to generate amino groups for conjugating HA and GQDs to prepare multifunctional nanoparticles. FT-IR spectrum of silica nanoparticles along with HA and GQDs functionalized MSNs showed characteristic peaks of silica along with the peak at 1680- 1700 cm-1 representing the formation of CO-NH2 (carbodiamide bond) that proved the successful functionalization of HA and GQDs on MSN (data not shown). Further characterization like zeta potential data was also evaluated and the results showed an increase in zeta potential towards negative side. The corresponding values of potential were -28.15, -29.84, - 32.37 mV for MSN and HA-MSNs and HA-GQDMSN that also confirmed the functionalization of MSN. The increase in the zeta potential was due to the negative charge on HA acid at physiological pH. [Figure S1] The negative charge on nanoparticles prevents their aggregation and also useful to prevent non specific binding to cell surface. The major advantage of this nanoformulation was its multifunctionality. The HA moiety gave better targeting potential and accumulation of nanoparticles on cancer cells due to improved endocytosis mechanism. HA binds with the CD44 cells that help internalization of nanoformulation via HA receptor mediated endocytosis. On the other hand the conjugation of GQDs on MSN made it a complete nanotheranostic system due to the bioimaging capabilities of the nanoformulation GQDs are the biocompatible nanosheets of graphene having excellent fluorescence properties. In general there are two primary approaches for synthesizing GQDs are there, that can be classified as bottom-up and top down methods and a variety of procedures have been reported based on these techniques for synthesis of GQDs. In top down methods involve cutting large graphene sheets where as in bottom up approach, quantum dots are synthesized from a carbon precursor containing conjugated carbon atoms. Although bottom up approach provides a precise control over size and morphology, the synthesis procedure is quite tedious and complicated. With regard to this, top down approaches are a better alternative with their simple methodology and mass production. In this analysis GQDs were prepared via hydrothermal reduction of Graphene oxide (GO) with Lawsone as earlier described by us [18] and were further characterized. Hydrothermal approach of synthesis is simple, versatile and has the potential of scalability. The synthesized particles exhibited excellent green fluorescence. Apart from the characteristic peak at 230 nm due to π- π* transition of C=C domains, UV-Vis spectrum of GQDs also showed an absorption peak at 340 nm [Figure S2]. The peak at 340 nm represents the uniform sp2 clusters in GQDs. An excitation dependent PL spectra was obtained from GQDs and two peaks at 430 nm and 540 nm were observed with 340 nm excitation whereas for 360-480 nm excitation, only one peak at 540 nm, responsible for green fluorescence of GQDs was obtained. Quantum yield of GQDs was ~14% calculated as per the method described by Zhu et al. at 360 nm excitation wavelength using 9,10- Bis (phenylethynyl) anthracene in cyclohexane (QY=1) as reference compound [32]. After functionalization, fluorescence peak intensity of GQDs got diminished but still it was sufficient and stable enough for bioimaging applications. TEM image revealed GQDs of average size 2-5 nm as illustrated in Figure 4. Gemcitabine was loaded on MSN via physical adsorption and it gets internalized into the pores of MSNs. MSNs have an open entrance for drugs to enter in with well-ordered network of channels for homogeneous distribution of drug molecules. The high surface area due to porous structure of MSNs provide better drug loading and sustain release properties. The drug loading efficiency of Gem on MSN was determined by UV-Vis spectroscopy [Figure 5] and approximately 90% loading was obtained for gem (Concentration 10 mg/ml). The nanoformulation also showed a good release behavior for gemcitabine with approximately 75 h of sustained release [Figure 6].
Cytotoxicity analysis of nanoformulation
Cytotoxicity of the nanoformulation was judged by MTT against Panc-1 cells. The cells were incubated with free gemcitabine, MSNGem, HA-MSN-Gem, HA-MSN, HA-MSN-GQDs and GQDs. The IC50 value for free gem was 0.96μg/ml but with HA functionalized nanoformulation a significantly reduced value of 0.41μg/ml was observed. The prime reason behind this decrease can be explained by the fact that HA conjugated MSN nanoparticles escaped the nonspecific binding and exhibited better internalization due to HA receptor (CD44) mediated endocytosis as compared to the unfunctionalized nanoparticles and free gemcitabine. More over the sustained release of gem from the silica nanoparticles was also a major factor for better anticancer properties of HA functionalized nanoformulation. MSN and MSN-HA, GQDs and HA-MSN-GQDs showed almost no toxicity with ~82-91% cell viability as shown in Figure S3. This data represents that the nanocarrier itself was not cytotoxic and the anticancer effect was due to encapsulated gemcitabine. It is a well known fact that gemcitabine has a short half life and rapid metabolism that curtails its therapeutic effects. But MSN loaded nanoformulation not only improved its efficacy due to sustained release but also provided a protective environment against external stimulations.
Cellular uptake of MSN formulation
To examine the uptake efficiency of the MSNs by cancer cells, they were incubated with HAMSNs- GQDs. The cells internalized with HA-MSNs-GQDs were observed by confocal imaging. HAMSNs were functionalized with GQDs for bioimaging considering the photoluminescence and surface grafting properties of GQDs. As clearly evidenced, in the absence of GQDs, only DAPI stained cells are visible but in presence of GQDs a strong (as exhibited in red color) was observed inside the cells. Based on the difference in fluorescence intensities of HA modified and unfunctionalized MSN in Figure 7, it can be concluded that uptake efficiency of HA-MSNs was higher than those of MSNs thereby confirming the clear role of HA (present on the external surface of MSNs) and CD44 receptor (over expressed on cancer cells) interaction in cellular internalization of MSNs.
Conclusion
In this study a multifunctional targeted drug delivery system has been developed based on hyaluronic acid and GQDs modified mesoporous silica nanoparticles. HA-MSNs possess a specific affinity towards CD44, a stem cell marker overexpressed on the surface of Panc-1 cell lines. The cellular uptake performance of GQD labeled MSNs with and without HA modification has been evaluated by confocal microscopy. Compared to bare MSNs, HA-MSNs exhibit a higher cellular uptake via HA receptor mediated endocytosis. An anticancer drug, gemcitabine (Gem) was loaded into MSNs and HA-MSNs. Gem loaded HA-MSNs show greater cytotoxicity to panc-1 cells than free Gem and Gem -MSNs due to the enhanced cell internalization behavior of HA-MSNs. This formulation has great potential to be further developed as an efficient nanocarrier for targeted delivery of anticancer drugs to CD44 over-expressing tumors.
Acknowledgement
The authors are grateful to the Department of Science of Technology, Government of India, for providing the necessary funding to meet the research expenses.
References
- Chu TC, Twu KY, Ellington AD, Levy M (2006) Aptamer mediated siRNA delivery. Nucleic Acids Res 34: e73.
- Khan DR (2010) The Use of Nanocarriers for Drug Delivery in Cancer Therapy. J Cancer Sci Ther 2: 58-62.
- Safari J (2014) Advanced drug delivery systems: Nanotechnology of health design A review. J Saudi Chem Society 18: 85-99.
- Piao Y, Burns A, Kim J, Wiesner U, Hyeon T (2008) Designed Fabrication of Silica-Based Nanostructured Particle Systems for Nanomedicine Applications. Adv Funct Mater 18: 3745-3758.
- Kim J, Lee JE, Lee J, Yu JH, Kim BC, et al. (2006) Magnetic Fluorescent Delivery Vehicle Using Uniform Mesoporous Silica Spheres Embedded with Monodisperse Magnetic and Semiconductor Nanocrystals. J Am Chem Soc 128: 688-689.
- Lee JE, Lee N, Kim H, Kim J, Choi SH, et al. (2010) Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc 132: 552-557.
- Vallet-Regi M, Ramila A, del Real RP, Perez-Pariente J (2001) A new property of MCM-41: drug delivery system. Chem Mater 13: 308-311.
- Angelos S, Yang YW, Patel K, Stoddart JF, Zink JI (2008) pH-Responsive Supramolecular Nanovalves Based on Cucurbit[6]uril Pseudorotaxanes. Angew Chem 120: 2254-2258.
- Chen C, Pu F, HuangZ, LiuZ, Ren J, et al. (2011) Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers. Nucleic Acids Res 39: 1638-1644.
- Chen Z, Li Z, Lin Y, Yin M, Ren J, et al. (2013) Bioresponsive Hyaluronic Acid-Capped Mesoporous Silica Nanoparticles for Targeted Drug Delivery. Chem Eur J 19: 1778-1783.
- Zhu YF, Fang Y, Kaskel S (2010) Folate-Conjugated Fe3O4@SiO2 Hollow Mesoporous Spheres for Targeted Anticancer Drug Delivery. J Phys Chem C 114: 16382-16388.
- Brevet D, Gary-Bobo M, Raehm L, Richeter S, Hocine O, et al. (2009) Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun 1475-1477.
- Jiao Y, Shen S, Sun Y, Jiang X, Yang W (2015) A Functionalized Hollow Mesoporous Silica Nanoparticles-Based Controlled Dual-Drug Delivery System for Improved Tumor Cell Cytotoxicity. Part Part Syst Char 32: 222-233.
- I. I. Slowing, P. A. Kapke, S. Goodison and V. S. Y. Lin, The 235th ACS National Meeting, New Orleans, LA, April 8, 2008.
- Pliarchopoulou K, Pectasides D (2009) Pancreatic cancer: Current and future treatment strategies. Cancer Treat Rev 35: 431-436.
- Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, et al. (2009) A compendium of potential biomarkers of pancreatic cancer. PLoS Med 6:e1000046.
- Merl MY, Li J, Saif MW (2010) The first-line treatment for advanced pancreatic cancer. Highlights from the "2010 ASCO Gastrointestinal Cancers Symposium Orlando, FL, USA. January 22-24. JOP 11: 148-150.
- Nigam P, Waghmode S, Louis M, Wangnoo S, Chavan P, et al. (2014) Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J Mat Chem B 2: 3190-3195.
- Papa AL, Basu S, Sengupta P, Banerjee D, Sengupta S, et al. (2012) Mechanistic studies of Gemcitabine-loaded nanoplatforms in resistant pancreatic cancer cells. BMC Cancer 12: 419-429.
- Hong SP, Wen J, Bang S, Park S, Song SY (2009) CD44-positive cells are responsible for resistance in pancreatic cancer cells. Int J Cancer 125: 2323-2331.
- Yu M, Jambhrunkar S, Thorn P, Chen J, Gu W, et al. (2013) Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells Nanoscale 5: 178-183.
- Almeida PV, Shahbazi MA, Mäkilä E, Kaasalainen M, Salonen J, et al. (2014) Amine-modified hyaluronic acid-functionalized porous silicon nanoparticles for targeting breast cancer tumors. Nanoscale 6: 10377-10387.
- Hummers WS, Offeman RE (1958) Preparation of Graphitic Oxide. J Am Chem Soc 80: 1339-1339.
- Chan JM, Valencia PM, Zhang L, Langer R, Farokhzad O (2010) Polymeric nanoparticles for drug delivery. Methods Mol Biol 624: 163-175.
- Cho K, Wang X, Nie X, Chen Z, Shin DM (2008) Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin Cancer Res 14: 1310-1316.
- Hild W, Pollinger K, Caporal A, Cabrele C, et al. (2010) G protein-coupled receptors function as logic gates for nanoparticle binding and cell uptake. PNAS 107: 10667-10672.
- Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9: 615-627.
- Bae YH, Park K (2011) Targeted drug delivery to tumors: myths, reality and possibility. J Controlled Release 153: 198-205.
- Lu J, Li, Z, Zink JI, Tamanoi F (2012) In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomed Nanotech Biol Med 8: 212-220.
- Lodha A, Lodha M, Patel A, Chaudhuri J, Dalal J, et al. (2012) Synthesis of mesoporous silica nanoparticles and drug loading of poorly water soluble drug cyclosporin A. J Pharm Bioallied Sci. 4: S92-94.
- Lin YS, Haynes C (2010) Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc 132: 4834-4842.
- Zhu S, Zhang J, Qiao C, Tang S, Li Y, et al. (2011) Strongly green photoluminescent graphene quantum dots for bioimaging applications. Chem Commun 47: 6858-6860.