Research Article
Bio-Removal of Chlorophenols from Industrial Effluents in Open Bioreactor System
Santosh Kr. Karn1*, Shweta Kumari2 and S. K. Chakrabarti2
Corresponding author: Dr. Santosh Kr. Karn, Key Laboratory of Marine Environmental Corrosion & Biofouling, Institute of Oceanology, Chinese Academy of Sciences, No. 7 Nanhai Road, 266071, Qingdao, China, Phone (M) +86-15092273512,E-mail: santoshkarn@gmail.com
Citation: Karn SK, Kumari S, Chakrabarti SK. Bio-Removal of Chlorophenols from Industrial Effluents in Open Bioreactor System. J Chem Applied Biochem. 2015;2(1): 108.
Copyright © 2015 Santosh Kr. Karn et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Submission: 03/01/2015; Accepted: 25/03/2015; Published: 31/03/2015
Abstract
Present study focused on the bio-removal of chlorophenols from the pulp and paper mill effluents which is a serious concern, for this work we used diluted black liquor containing 12 different chlorophenols and having high absorbable organic halogen (AOX), extractable organic halogen (EOX) and chemical oxygen demand (COD). Bio-removal of the chlorophenols was evaluated in open reactor system using consortium of five efficient bacterial strains isolated from the sludge of same industry, Gas chromatography (GC) analysis confirm that these isolate removed more than 95% of 12 different chlorophenols [2,4,6-trichlorophenol (2,4,6-TCP); 2,4,5-trichlorophenol (2,4,6-TCP); 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP); 3,4,6-trichloroguacol; 3,4,6-trichlorocatecol; 3,4,5-trichlorocatecol (3,4,5-TCP); Tetrachloroguaiacol; Tetrachlorocatecol; Trichlorosyringol; Pentachlorophenol (PCP); 3,4,5-trichloroguacol; 4,5,6-trichloroguacol] which are under regulation of environmental protection agency (EPA) in the wastewater to less than detection level,. Simultaneously AOX removal was 82%, EOX 65% and COD 92% was observed. Present result shows the high efficiency of the consortium for the pulp and paper mill effluents. Finally these isolate were identified using 16s rRNA gene sequencing which revealed that Rhodococcus sp. (SS1), Aeromonas sp. (SS2) Chryseobacterium sp. (SS3) Uncultured bacterium clones (SS4) and Pseudomonas sp. (SS5). Further this consortium was found to be noble and can be used for the removal of pollutants from the industrial effluents.
Keywords: Bioreactor; Chlorophenols; Organic pollutants, Aeromonas sp., Chryseobacterium sp.; Pulp and Paper industry
Introduction
Pulp and paper industry being categorized under the red category polluting industries, it has been under tremendous environmental pressure, to improve the performance to decrease the pollution level. Water consumption being large and the effluents volume generated are also large. The effluent treatment processes adopted guarantee the achievement of parameter values below the pollution control board norms, including specific effluent discharge. Different chlorinated orchlorolignin derivatives formed during Kraft pulp bleaching, such as chloroguaiacols, chlorophenols, chlorocatecols and some chloroaliphatics are extremely toxic and recalcitrant compounds are generated from pulp bleaching process, that accumulate in sediments or at different levels of the trophic chain. When discharged into aquatic systems, they are responsible for detrimental effects towards all life forms [1]. The United states environmental protection agency (EPA) [2], regulates twelve different chlorophenolic substances 2,4,6-trichlorophenol (2,4,6-TCP); 2,4,5-trichlorophenol (2,4,6-TCP); 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP); 3,4,6-trichloroguacol; 3,4,6-trichlorocatecol; 3,4,5-trichlorocatecol (3,4,5-TCP); Tetrachloroguaiacol; Tetrachlorocatecol; Trichlorosyringol; Pentachlorophenol (PCP); 3,4,5-trichloroguacol; 4,5,6-trichloroguacol in the wastewater to less than detection level, among all the chlorophenols PCP in surface and ground water and soil is a major environmental concern [3] because PCP is very toxic and dangerous compounds in nature. PCP is toxic to all life forms as it is an inhibitor of oxidative phosphorylation in plants [4]. A number of methods such as oxygenolysis, hydroxylation or reductive dehalogenation process have been used to eliminate chlorophenols from industrial effluents, but none of these methods or their combinations have achieved complete mineralization of chlorophenols [5,6]. Although chemical methods are efficient, they may generate undesirable byproducts, besides being very expensive. By contrast, biological methods are generally more efficient and low-costthan chemical methods. Biological depletion is extremely attractive for waste treatment processing because microorganisms mineralize a variety of compounds [7,8]. Microorganisms showed excellent ability at tolerating and removing PCP, by converting it into a non-toxic compound or by using PCP as the sole source of carbon [5]. However, the chlorophenolic removal rates of reported bacterial species have been found to be not efficient for this reason there is still need for continued search of more efficient bacterial strains for bioremediation of chlorophenols from effluents of pulp and paper mill. Therefore we choose PCP which is more toxic and having highest chloride ion, as carbon source to isolate the resistant bacteria, keeping in view the bacterium able to grow on this, can be able to utilize other chloride containing compounds. This paper has focused on the removal of 12 different chlorophenols and organic pollutant in open bioreactor system from effluents of pulp and paper mills by using consortium of five efficient bacterial strains.
Material and Methods
Sample collection
Pulp paper mill effluent sample were collected from M/s Shree Gopal Unit (BILT) pulp paper mill, Yamunanagar, Haryana, India. This industry adopts Kraft process for pulping and the bleaching sequence in the mill is CDEOPD1D2; 20% chlorine dioxide substituted chlorine stage, extraction stage with oxygen and peroxide reinforced, two stage chlorine dioxide bleaching in the last stages. It is true that generation of chlorophenolic compounds depends upon the bleaching sequence, particularly on the usage of chlorine in the first stage. In the present case all the chlorophenolic compounds that have been detected are due to the above reason. Diluted black liquor from a kraft pulp plant was used as a basal substrate in the reactors because black liquor as the basic component of synthetic effluents has already been used by other researchers and has proven appropriate for laboratory studies of effluent treatment for pulp and paper mills [9,10].
Microorganism
Bacterial strains were isolated from sludge of pulp and paper mill by enrichment method in mineral salt medium (MSM), supplemented with PCP 3 mM as sole source of carbon and energy. The MSM contained the following components at the specified concentrations (in mg/L): KH2PO4, 800; Na2HPO4, 800;MgSO4.7H2O, 200; CaCl2.2H2O, 10; NH2Cl, 500; plus 1 mL of trace metal solution which includes FeSO4.7H2O, 5; ZnSO4.7H2O, 4; MnSO4.4H2O, 0.2; NiCl.6H2O, 0.1; H3BO3, 0.15;CoCl2.6H2O, 0.5; ZnCl2, 0.25; and EDTA, 2.5. PCP was added to the medium after being sterilized by syringe filter through a 0.2 μm membrane. Bacteriological agar at 1.5% (w/v) was added for MSM plate preparation. The bacterial culture maintained at 37 ± 2°C.
Bioreactor
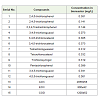
The bioreactor designed on laboratory scale was fabricated by using glass fibers (size 28 x 13 cm) having 10 liter capacity but the effluent volume used for treatment was 6 liter. It has an outlet, present in the uppermost part of the reactor, and an outlet at lower part of but it was kept closed. The upper part outlet was connected to primary clarifier and further this is connected with secondary clarifier. From secondary clarifier treated effluents were discharged. The stirring of the effluent was made possible by fixing a motor. The motor speed was 120 rpm per min, oxygen was provided by passing air through an air pump. Inside the bioreactor following condition was maintained, dissolved oxygen (DO) 1±0.5 mg/L, Temp 35˚C, flow rate 8.1 /hr, COD 1400 mg/L, AOX 2500 mg/L, EOX 800 mg/L and initial load of different concentration of 12 different chlorophenols viz 2,4,6-trichlorophenol (2,4,6-TCP); 2,4,5-trichlorophenol (2,4,5-TCP); 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP); 3,4,6-trichloroguacol; 3,4,6-trichlorocatecol; 3,4,5-trichlorocatecol (3,4,5-TCP); Tetrachloroguaiacol; Tetrachlorocatecol; Trichlorosyringol; PCP; 3,4,5-trichloroguacol; 4,5,6-trichloroguacol given in (Table 1). 1.5% (106 CFU/mL) microbial consortia of 5 efficient strains were added as inoculums when OD was more than 0.5. Reactor was continuously operated for 7 d; sample was collected at every 24 hr to check the microbial growth and released chloride ion. Control experiments were set up in parallel to estimate the levels of chlorophenols and organic pollutant without microbial inoculants.
Determination of Chloride release assay, COD, AOX and EOX
Bacterial biomass was measured in term of increase in the form of colony forming units (CFU). It was determined by taking 1 mL of the effluent sample and plating them on nutrient agar plates by serial dilution method to count the CFU. Chloride ion released in the effluents was determined at every 24 hr of interval up to 7 d using 5 mL of culture filtrate, using Orion ion analyzer model 940 (NyCo Systems, USA) with calibrated chloride ion selective electrode. Chloride concentration was determined using a calibration curve plotted from the log of chloride molarity for a series of standard samples ranging from 10 to 1000 mg/L. COD was determined by dichromate reflux method APHA [11] in this method, the sample was refluxed with potassium dichromate and sulphuric acid, and titrated with ferrous ammonium sulphate. The AOX content was estimated as per DIN [12] with little modification. EOX content in sludge was estimated as per EPA method 9023 [13] by using Euroglas Netherlands instrument ECS-2000 according to the manufacturer’s recommended procedure.
Estimation of chlorophenols
Removal efficiency chlorophenols was determined from the treated effluents of secondary clarifier, sample collected on 1st 3rd 5th and 7th day of treatment. In extraction of chlorophenols, the effluents were clarified by centrifugation at 8,000 rpm for 5 min. The free supernatant cells fractions were extracted three times with an equal volume of n-hexane by shaking vigorously for 15 min in a standard separating funnel. The organic layer was dried with anhydrous sodium sulfate. The residue was finally dissolved in a 50 μL mixture of n-hexane: ethyl acetate (10:1) and analyzed immediately on a GC. The GC analyses were performed in electron capture detection mode with a gas chromatograph Nucon GC-5765 (Centurion Scientific, India) capillary column DB-5 (30 meter length 9 0.025 mm i.d. 9 0.25 Lm film thickness) was used at a temperature program of 50 °C (2 min), then raised to 10 °C /min to 280 °C, where it was held for 10 min. Helium was used as the carrier gas at a constant flow of 1.2 mL/min. The samples were analyzed in splitless mode at an injection temperature of 250°C, and detector temperature of 280 °C, The injected volume was 0.1 μL.
Identification of bacterial isolates
Total DNA was extracted as per standard protocol from overnight grown cultures. The 16S rRNA gene was amplified from 1 μL purified genomic DNA using a polymerase chain reaction (PCR) as described by Karn et al. [14]. The amplified product was gel purified using QIA gel extraction kit, Qiagen, USA, and sequenced at DNA sequencing facility, Delhi University, India .The sequence data was analyzed by BLAST analysis and identified based on the closest identity of reported sequence data. The sequences were deposited in GenBank database (http://www.ncbi.nlm.nih.gov/) under accession no. KF278595 (SS1), KF278599 (SS2), KF278596 (SS3), KF278597 (SS4) and KF278598 (SS5). Phylogenetic tree were constructed using the Mega (v 5.1) software. Sequences or construction of the trees were downloaded from the NCBI GenBank site.
Data analysis
Data were analyzed by using ANOVA (Analysis of variance) software and the means were compared by Tukey-Kramer Multiple Comparison Test at p < 0.05. Three replicates were used for each study. All the analyses were performed using GraphPad Prism (v 4.03) software.
Results and Discussion
Removal of chlorophenols and organic pollutant in bioreactor
Environmental concerns coupled with governmental regulation have prompted research on the environmental friendly of industrial effluent. Pulping process utilizes large amounts of water, which reappear in the form of an effluent. The most significant sources of pollution among various process stages are wood preparation, pulping, pulp washing, screening, washing, bleaching, and paper machine and coating operations. Among the processes, pulping generates a highstrength wastewater especially by chemical pulping. The effluent discharged from pulp and paper mill carries a high COD load due to the presence of organochlorine compounds generated during the pulping and bleaching processes. These organic compounds in the effluents are degradation products of lignin. Among these, tri, tetra, and pentachlorophenol, chlorinated catechols, chlorinated guaicols and dioxins are of particular importance, as they are known to be highly recalcitrant, and responsible for colour, mutagenicity, carcinogenicity and toxicity of the effluent [15]. Removal of these pollutants is of great importance it was observed that the bioreactor treatments by aerobic microorganisms showed a reduction in pollution load significantly. In the present study we also found that aerobic treatment in the bioreactor has made significant in the removal of chlorophenolic compounds from 3rd day and 5th of the treatment (Table 2) the reason behind this microbial consortia initially adapted and start to multiply further depletion of the chlorophenols was observed. The survival of microbial consortia was monitored by determining the growth in the form of CFU (Figure 1). The number of cells increased in the effluents showing that microorganism luxuriously able to grow by utilizing organic contaminants from the effluents without addition of any extra nutrients. However, numerous studies have demonstrated that under aerobic conditions, chlorophenols can be efficiently reduced until a complete mineralization [7,16-18]. Previously remediation of pulp and paper mill sludge has been conducted by Karn et al. [14] using Kocuria sp. grown well and able to remove up to 70% of the PCP from the sludge contaminated with 100 mg/L. Melin et al. [19] conducted in fluidized bed reactors evaluated the treatment of lower concentrations of chlorophenols, characteristic of contaminated groundwater. Degradation of xenobiotic compounds is believed to more rapidly and efficiently degrade by the aerobic bacteria. The indigenous bacteria derived from the same environment or sludge used in this study showed sufficient growth abilities. Removal of organic pollutants was monitored by checking chloride ion from the effluents, Chloride ion release was increased gradually and attained highest concentration of 0.5 mM within 5 d, with a slight variation in pH (ranging from 7.8 to 7.2) (Figure 2). Although there was a positive correlation between chloride release and chlorophenols removal, the relationship was very obvious as the chloride released could have been contributed by the chlorophenols. Previous report also suggested that degradation of chlorophenols by other bacteria isolates and release of chloride ion in to the medium [20]. Chu and Kirsch [21] described a bacterial strain (KC3) capable of mineralizing [14C]-PCP to 14CO2 when supplied as a sole carbon source. Polychlorinated phenols (3 to 5 chlorines) are converted to chlorohydroquinones as the initial intermediates (hydroquinone pathway). Subsequent reactions are known to progressively remove chlorines from the ring prior to ring cleavage [22]. On the other hand, this may also be done by means of hydroxylation reactions which convert PCP into other compounds such as tetrachlorohydroquinone (TCHQ) [17,18] by replacing the chlorine atom with a hydroxide, converting the PCP into intermediate products [17,18].
High amount of AOX and EOX values were observed in effluents samples possibly due to usage of higher amount of elemental chlorine in the bleaching process. Different organochlorine contaminants like Adsorbable organic halides (AOX) and Extractable organic halides (EOX) in the effluents were analyzed (Table 1). AOX values decreased from 2856 mg/L to 515 mg/L in the effluents where as EOX values decreased from 800 mg/L to 280 mg/L results showed that microbial consortium able reduced the high level of AOX 82% and EOX 65% as compre to control sample. Earlier studies on the treatment of the paper mill effluents using white-rot fungi have reported high removal efficiency for AOX and EOX from Kraft pulp bleaching were 32% and 36%, respectively by incubation by Ceriporiopsis subvermispora CZ-3 [23,24]. Present result showed that bacterial strain in microcosm form removed greater extent or high percentage of AOX as well as EOX, which is of great importance.
Table 2 presents the mean chlorophenolic compounds removal values in the bioreactor system and the chlorophenols concentration in control reactor (without microbial inoculants) after the experimental period. It can be seen that the removal in the bioreactor was superior, the average individual values for organochlorine removal efficiency in bioreactor were: 99.9% for the 4,5,6-trichloroguacol; 3,4,5-trichlorocatecol; 3,4,6-trichloroguacol, 97% for the 3,4,5-trichloroguacol; Tetrachlorocatecol, 95% for the 2, 4, 5-TCP Tetrachloroguaiacol and PCP. Correa et al. [23] observed that after an acclimatization period, removal efficiency of 2,4,6-TCP during aerobic process was always above 95%. Present microbial consortia were able to remove COD from 1200 mg/L to 132 mg/ L approximately 92% removal efficiency was observed in the reactor. In the recent studies aerobic processes have been recently used for the treatment of textile wastewater as standalone processes and it is confirmed that they are efficient and cost effective for smaller molecules. Various researchers Coughlin et al. [24]; Khehra et al. [25]; Buitron et al. [26]; Sandhaya et al. [27] found the use of an aerobic reactor is an effective technique to treat industrial wastewater. Various researcher Kornaros & Lyberatos [28]; Balan & Monteiro [29] affirm that among low cost, viable alternatives, available for effluent treatment and decolorization, the biological systems are recognized by their capacity to reduce biochemical oxygen demand (BOD) and COD through conventional aerobic biodegradation. Therefore present studies suggest that indigenous microbes are adapted for particular environment can be exploited for the remediation of site contaminated in the environments using a bioaugementation as an option. The addition of pollutant degrading microorganisms is most likely to succeed in environmental cleanup when the introduced organisms are active and competitive. This needed to be worked out at biochemical and genetic level. Considering the degradation potential of all isolates, it can be used as a consortium to decontaminate the chlorophenolics contaminated site in the environment.
Microorganism-identification and phylogenetic relationship
Bacterial strains were isolated by enrichment method using pentachlorophenol as a sole carbon or energy sources. Based on their potential to grow on PCP five isolates were selected for further study and designated as SS1, SS2, SS3, SS4 and SS5. All the selected isolate were able to grow at high concentration up to 3 mM of PCP. Further identification of the isolates were done by 16s rRNA gene sequencing. Though 16S rRNA gene is found conserved on evolutionary scale, it is still diverse enough to identify and classify the eubacteria [30]. The 16s rRNA gene sequence prepared for BLAST and RDPII analysis showed that SS1, shown close identity to Rhodococcus sp. family Nocardiaceae and belong to phylum Actinobacteria; SS2 Aeromonas sp. belong to family Aeromonadaceae, phylum Proteobacteria; SS3 Chryseobacterium sp. family Flavobactericeae and phylum Bacteroidetes; SS4 Uncultured bacterium clones family Enterobactericeae and phylum Proteobacteria whereas SS5 Pseudomonas sp. family Pseudomonadaceae and phylum Proteobacteria. Five sequences included in the dataset retrieved from NCBI, to create phylogenetic tree which shown in (Figure 3). Present result was supported by previous finding of Apajalahti & Salkinoja- Salonen [31,32] reported Rhodococcus chlorophenolicus able to degrade four different chlorophenols like 2,3,5-trichlorophenol (2,3,5-TCP), 2,3,6-trichlorophenol (2,3,6-TCP), 2,3,4,5-tetrachlorophenol (2,3,4,5- TeCP) and PCP with limited concentration. Goswami et al. [33] also found that Rhodococcus erythropolis able to utilize 2, 4-dichlorophenol (2, 4-DCP). This shows that Rhodococcus sp. has potential to utilize chlorophenols as a carbon sources. The degradation of a mixture of phenol was studied by Buitrón et al. [26] 4-chlorophenol (4-CP), 2,4-dichlorophenol (2,4-DCP) and 2,4,6-trichlorophenol (2,4,6-TCP) in acclimated activated sludge and by Aeromonas sp., Pseudomonas sp. and Chryseomonas luteola. Activated sludge was acclimated for 70 d to 40 mg phenols per liter then the microorganisms responsible for the chlorophenol degradation. Present isolates having abilities to degrade wide range of chlorophenols. When new organisms have been isolated with high biodegradation efficiency, their biochemical versatility has been found to be immense. Attempts to determine microbial diversity in natural environments like soil are limited by the inability of the microbiologists to culture specific microbes present in a particular environmental sample. However, the isolation of those microbes will often require a targeted intelligent approach to screen the biosphere for its presence [20]. Strain SS5 belongs to genus Pseudomonas is widely applied for the degradation of phenolic compounds. These bacteria are known for their immense ability to grow on various organic compounds. Some previous reports (Radehaus & Schmidt [34]; Shah & Thakur [35] also showed the degradation of PCP by other Pseudomonas species. Strain SS4 shown closet identity with uncultured bacterium clones and Enterobacter sp. sequences. Recently Karn et al. [36] found Enterobacter sp. were able to remove PCP up to 2 mM concentration. Furthermore, bacterial consortium showed the greatest efficiency in regards to, removal of all 12 different chlorophenols in the bioreactor which is under the regulation USEPA.
Acknowledgement
Authors are thankful to Dr. S. K. Chakrabarti, Director TCIRD Yamunangar, India for providing the effluents and black liquor during the course of study.
References
- Yeber MC, Freer J, Martinez M, Mansilia HD (2000) Bacterial response to photocatalytic degradation of 6-chlorovanilin. Chemosphere 41: 1257-1261.
- Environmental Protection Agency (1995) EPA office of compliance sector notebook project: profile of pulp and paper industry. Washington DC 20460, USA: EPA/310-R-95-015.
- Wu ZY, Cong Y, Zhou M, Ye Q, Tan T (2002) Removal of Phenolic Compounds by Electro-assisted Advanced Process for Wastewater Purification. Korean J Chem Eng 19: 866-870.
- Yang CF, Lee CM, Wang CC (2006) Isolation and physiological characterization of the pentachlorophenol degrading bacterium Sphingomonas chlorophenolica. Chemosphere 62: 709-714.
- McAllister KA, Lee H, Trevors JY (1996) Microbial degradation of pentachlorophenol. Biodegradation 7: 1-40.
- Field AJ, Sierra-Alvarez R (2008) Microbial degradation of chlorinated phenols. Rev Environ Sc Biotechnol 7: 211-241.
- Rubilar O, Diez MC, Gianfreda L (2008) Transformation of chlorinated phenolic compounds by white rot fungi. Crit Rev Environ Sci Technol 38: 227-268.
- Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9: 215-288.
- Springer AM (2000) Industrial Environmental Control: Pulp and Paper Industry, 3rd ed. Tappi Press. Atlanta.
- Buzzini AP, EP Gianotti, EC Pires (2005) UASB performance for bleached and unbleached kraft pulp synthetic wastewater treatment. Chemosphere 9: 55-61.
- 11. APHA (1995) Standard methods for the examination of water and waste water. 19th ed. Washington, DC: American Public Health Association. ISBN 08755-32233.
- DIN (1989) Bestimmung adsorbierbarer organisch gebundener Halogene (AOX), DIN 38414 S18 Deutsche Einheitsverfahren zurWasser-,Abwasser und Schlammuntersuchung Schlamm und Sedimente (Gruppe S) Beuth, Berlin.
- EPA (1996) Method 9023, Extractable organic halides in solids. 1-8.
- Karn SK, Geetanjali (2014) Pentachlorophenol Remediation by Enterobacter sp. SG1 Isolated from Industrial Dump Site. Pak J Biol Sci 17: 388-394.
- Abassi A (1985) Occurrence, toxicity and treatment of lignin in pulp and paper effluents. J Environment 65: 1-8.
- Reddy GV, Gold MH (2000) Degradation of pentachlorophenol by Phanerochaete chrysosporium: intermediates and reactions involved. Microbiology 146: 405-413.
- Crawford RL, Jung CM, Strap JL (2007) The recent evolution of pentachlorophenol (PCP)-4-monooxygenase (PcpB) and associated pathways for bacterial degradation of PCP. Biodegradation 18: 525-539.
- Xun L, Belchik SM, Xun R, Huang Y (2010) S-Glutathionyl-(chloro)hydroquinone reductases: a novel class of glutathione transferases. Biochem J 428: 419-427.
- Melin ES, Puhakka JA, Ferguson JF (1998) Enrichment and operation strategies for polychlorophenol degrading microbial cultures in an aerobic fluidized-bed reactor. Water Environ Res 70: 171-180.
- Wackett LP, Hershberger DC (2001) In Biocatalysis and Biodegradation, Microbial transformations of organic compounds. ASM press, Am Soc Microbiol Washington DC.
- Chu JP, Kirsch EJ (1972) Metabolism of pentachlorophenol by an axenic bacterial culture. App Microbiol 23: 1033-1035.
- Sahoo DK, Gupta R (2005) Evaluation of ligninolytic microorganisms for efficient decolorization of a small pulp and paper mill effluent. Process Biochem 40: 1573-1578.
- Correa JVM, Dominguez VM, Martinez, Vidal G (2003) Aerobic degradation of 2,4,6-TCP content in ECF bleached effluent. Environ International 29: 459-465.
- Coughlin MF, Kinkle BK, Bishop PL (2002) Degradation of acid orange7 in an aerobic biofilm. Chemosphere 46: 11-19.
- Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigments 67: 55-61.
- Buitrón GA, González, Luz M, López-Marí (1998) Biodegradation of phenolic compounds by an acclimated activated sludge and isolated bacteria. Water Sc Technol 37: 371-378.
- Sandhaya SS, Padmavathy K, Swaminathan Subrahmanyam YV, Kaul SN (2005) Microaerophilic-aerobic sequential batch reactor for treatment of azo dyes containing simulated wastewater. Process Biochem 40: 885-890.
- Kornaros M, Lyberatos G (2006) Biological treatment of waste waters from a dye manufacturing company using a trickling filter. J Hazard Mater 136: 95-102.
- Balan DSL, Monteiro RT (2001) Decolourization of textile indigo dye by ligninolytic fungi. J Biotechnol 89: 141-145.
- Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cell without cultivation. Microbiol Rev 59: 143-169.
- Apajalahti JHA, Salkinoja-Salonen MS (1986) Degradation of polychlorinated phenols by Rhodococcus chlorophenolicus. Appl Microbiol Biotechnol 25: 62-67.
- Apajalahti JH, Salkinoja-Salonen MS (1987) Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J Bacteriol 169: 675-681.
- Goswami M, Shivaraman N, Singh RP (2002) Kinetics of chlorophenol degradation by benzoate-induced culture of Rhodococcus erythropolis M1. World J Microbiol Biotechnol 18: 779-783.
- Radehaus PM, Schmidt SK (1992) Characterization of a novel Pseudomonas sp. that mineralizes high-concentrations of pentachlorophenol. Appl Environ Microbiol 58: 2879-2885.
- Shah S, Thakur IS (2002) Enrichment and characterization of microbial community of tannery effluent for the degradation of pentachlorophenol. W J Microbio Biotechnol 18: 693-698.
- Karn SK, Chakrabarti SK, Reddy MS (2011) Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation 22: 63-69.