Research Article
A Thermodynamic Study of Charge Transfer Complexes of C60 and Some MacrocyclicLigands
Shehadeh Mizyed, Ibtisam Aleteiwi and Deeb Marji*
Corresponding author: Dr. Deeb Saad Deeb Marji, Department of Chemistry, Yarmouk University, Irbid, Jordan,; E-mail: Deeb@yu.edu.jo
Citation: Mizyed S, Aleteiwi I, Marji D. A Thermodynamic Study of Charge Transfer Complexes of C60 and Some Macrocyclic Ligands. J Chem Applied Biochem. 2014;1(2): 106.
Copyright © 2014 Deeb Marji et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Chemistry & Applied Biochemistry | Volume: 1, Issue: 1
Submission: 24/09/2014; Accepted: 31/10/2014; Published: 01/11/2014
Abstract
4 and Schiff base crown ethers 5 and 6 was studied in toluene using UV/Vis spectrophotometry at five different temperatures. The values of stabilityconstant (Kc) for each complex were determined using Benesi-Hildebrand equation.
The obtained Kc values were found to depend on the cavity size, the macrocyclic effect and the substituents on the host. All complexes were found to have 1:1 stoichiometry as obtained by Job’s plot method. Inaddition, the enthalpy and the entropy of complexation were estimated for all complexes. The complexation between C60 and Ligands 1, 2 and 4 are enthalpy favored and entropy unfavored, while the complexation with 3, 5and 6 are enthalpy unfavored and entropy favored.
Keywords: Charge transfer, C60, Stability constant, Macrocyclic ligand,Thermodynamic parameters.
Introduction
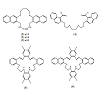
Macrocyclic Ligands are Polydentate cyclic compounds which contain donor atoms such as N, O, Se or S. These Ligands are very useful in supramolecular chemistry, as they provide suitable cavities that can completely surround different guest species including cations, anions or neutral molecules such as fullerene [1,2].
Fullerene (C60) is the third form of carbon after diamond and graphite. It was reported that C60 forms charge transfer complexes with a variety of host systems such as calixarenes [3,4], crown ethers [5], amines [6] and cryptands. These complexes have wide applications in many technological fields, such as in non-linear optical materials, surface chemistry [7], solar energy storage [8] and semiconductors [9].
The complexations between C60 and different types of crown ethers [10,11], thiacrown ethers [12] and selenacrown ethers [13] were extensively studied. The results of these studies show that C60 formed 1:1 complexes with these crown ethers. Several factors were reported to affect the stability of these complexes including the type and number of donor atoms, as well as the substituents and the cavity size of the crownethers. Several studies show that C60 formed 1:1 or 1:2 Charge transfer complexes with calix[n]arene and calixarene derivatives [14,4].
The present study was carried out to synthesize ligand 4, resynthesize ligand 6 and study the thermodynamic parameters of the charge transfer complexation of C60 with the macrocyclic ligands 1-6.
Experimental
C60 (99.0% Aldrich) and Toluene were used without any purification. Ligands (1-3, 5 and 6) were previously prepared according to the literatures [15,16,17].
The 1H-NMR and 13C-NMR spectra were recorded on 400 MHz and 100 MHz respectively, using Bruker Avance III. Melting points were determined using an electrothermal-digital melting point apparatus. Mass spectra were determined by VG-Analytical model 7070E.The absorption measurements were obtained using UV/Vis spectrophotometer “UV 2450-Shimadzu” connected to an electrical temperature controller with uncertainty ±0.1oc.
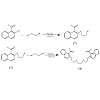
Preparation of compound (4)
Acyclic thiacrown ether (4) was prepared according to Scheme 1:
Compound 7 was synthesized according to the previously published procedure [17]. The physical and spectroscopic data were similar to the published data [17].
In 100 ml round bottom flask equipped with a magnetic stirrer and a reflux condenser, o-(2-bromoethoxy) naphthaldehyde 7 (0.82 g, 2.94 mmol), ethane-1,2-dithiol (0.12 ml, 1.47 mmol) and anhydrous K2CO3 (0.41 g, 2.94 mmol) were mixed with anhydrous CH3CN (60 ml). Themixture was refluxed for 24 hr under nitrogen atmosphere and then cooled to room temperature, filtered from residual K2CO3. Compound 4 was obtained as a solid product with an off-white color, m.p (147-148oc).
1H-NMR (CDCl3, 400 MHz) δ: 2.93 (4H, s, SCH2CH2S), 3.06 (4H, t, J=6, SCH2CH2O), 4.41 (4H, t, J=6, SCH2CH2O), 7.26 (2H , d, J=7,ArH), 7.44 (2H, J=7, t, ArH), 7.64 (2H, t, J=8, ArH), 7.77 (2H, d, J=8, ArH), 8.03 (2H, d, J=8, ArH), 9.26 (2H, d, J=8, ArH), 10.94 (2H, s, CHO).
13C-NMR (CDCl3, 100 MHz) δ: 31.4, 33.0, 69.3, 113.5, 117.2, 124.9, 125.0, 128.3, 128.8, 130.0, 131.5, 137.6, 162.8, 191.9.
DEPT 135 (CDCl3, 100 MHz) +ve: 113.5, 124.9, 125.0, 128.3, 130.0, 137.6, 191.9 . -ve: 31.4, 33.0, 69.3.
MS (m/z) = 491 M+.
The concentration of C60 in each solution was kept constant at (1.33* 10-5 M with ligands 1-4 and 1.00* 10-5 M with ligands 5 and 6). The concentration of the ligands changes from 0.00 up to 5*10-4 M. The stability constants were evaluated at different temperatures between 15 and 35oC using Benesi-Hildebrand equation. The thermodynamic parameters were determined using van’t Hoff equation.
The stoichiometry of the complexes was determined using Job’s plot method in which the total concentration of ligands and C60 is kept constant and the concentration of each is varied. Toluene was used as a solvent in all preparations.
Each solution was kept at the desired temperature for three days to attain the equilibrium. After that, the absorbance of each solution wasmeasured against toluene using a pair of matched quartz cell.
Results and Discussions
When a colorless solution of diaza crown ethers (1- 3) or acyclic crown ether 4 is mixed with violet C60 solution, the color changed to a pale yellow. This change in color of C60 may be due to the formation of charge transfer complexes between these ligands and C60. However,when a solution of Schiff base crown ether 5 or 6 is mixed with C60 solution, the yellow color does not appear which is probably due to the low concentrations of C60 and these ligands inspite of the change observed in UVVis spectra.
The absorption spectra of a series of solutions with ratios from 0 to 20 of ligand 1 to C60 were shown in Figure 1.
Figure 1 reveals the formation of a new absorption band at wavelength λ=315 nm, which shows blue and narrowing shifting of the peaks at λ=330 nm. The same results were obtained with ligands 2 and 3.
The absorption spectra of a cyclic crown ether 4 shows the appearance of two new absorption bands at λ=306 nm and 321 nm as shown in Figure 2. Several new bands in the range of 293-330 nm were observed in the absorption spectra of C60: Schiff base crown ether 5 complex as shown in Figure 3.
The change in C60 spectra of the complex formation with Schiff base crown ether 6 is shown in Figure 4. This spectra shows the broadening of the peak at λ= 335 nm.
The stoichiometries of all these complexes were found to be 1:1 using Job’s plot method as shown in Figure 5. This figure shows a maximum ratio at 0.5, indicating a 1:1 ratio for the complexes.
The stability constant values, KC, for the 1:1 complexes were determined using Benesi-Hildebrand equation 1:
Where [Aâ‚’] and [Dâ‚’] indicate the initial concentration of A and D respectively, δA the absorbance change and É› is the molar extension coefficient. When [Aâ‚’]/δA is plotted against1/[Dâ‚’] gives a straight line with a slope of 1/É›.Kc and an intercept of 1/É› is obtained, as shown inFigure 6.
The thermodynamic parameters δHâ—¦ and δSâ—¦ of the complex were obtained by applying the van’t Hoff equation 2:
A plot of lnKc vs 1/T gives a straight line with a slop of -δHâ—¦/R, and δSâ—¦/R as an intercept as shown in Figure 7. The values of log Kc, δHo, δSo and δGo are listed in the Table 1.
As shown in Table 1, the values of Log Kc between C60 and ligands 1, 2 and 3 decrease in order of 2 > 1 > 3. This behavior of the ligands can be explained by considering the cavity sizes of the ligands. It seems that ligand 2 has a cavity that better fits C60 molecule while the cavity of ligand 1 is smaller and cavity of ligand 3 is larger than C60 molecules. Previous study on the complexation of these ligands with Ni+2 showed that the complexes stabilities follow the sequence: 1 > 2 > 3. This was explaind depending on the cavity size matching too [16]. Since ligand 1 has smaller cavity size than 2, it formed a less stable complex with C60 resulted from the miss matching between the cavity size of 1 and C60. Ligand 3 has the weakest interaction with C60, this is probably due to that it has larger cavity size than 1 and 2 and more flexible than both, this flexibility requires preorganization step which needs a lot of energy to occur, also the orientation of the nitrogen atoms in the ring may be unsuitable for the complexation. Also, C60:4 complex has the lowest value of Log Kc, probably due to the “Macrocyclic Effect” which states that a macrocyclicligand complex is more stable than its open chain analogue.
The charge transfer complex of C60:6 is more stable than that of C60:5 as evidenced by larger Kc value. This is probably due to the presence of naphthyl group which afford extra binding sites for π-π interaction in comparison with phenyl group in 5.
The results in Table 1 also show that ligands 1, 2 and 4 have negative values of δHâ—¦ which indicate that these complexations are exothermic and enthalpy favored in toluene. However, δSâ—¦ values are negative indicating that these processes are endothermic and entropy unfavored.Considering ligands 3, 5 and 6, both δHâ—¦ and δSâ—¦ values are positive and relatively high, which indicates that the complexation between C60 and these ligands are enthalpy unfavored but entropy favored. It is known that when having stable complexes with strong interactions, formation of these complexes is enthalpic favered and entropic disfavored and vice versa.
Enthalpy-Entropy compensation effect
Table 1 shows that the values of δHâ—¦ are correlated to the values of δSâ—¦ and the regression of the equation 3 shows a good correlation when TδSâ—¦ is plotted against δHâ—¦ as shown in Figure 8.
The plot shows a good correlation with R2= 0.9954. The intercept is 15.64 and 0.99 as slope. This suggests that the entropic change consists of two components. This first is independent of the enthalpy change (TδSâ—¦) and the second is proportional to (αδHâ—¦), the proportionalityfactor might be considered as a quantitative measure of the enthalpy-entropy compensation. The positive intercept value indicated that the complexation is favored in the absence of an enthalpic contribution. This emphasizes that the complex formation is entropy favored while isenthalpy unfavored.
Previous studies showed enthalpy-entropy compensation effect. The complexes of thiacrown ether and calixcrown ethers with I2 showed enthalpy-entropy compensation with 0.8 correlation coefficient [18]. This was explained that the entropic change consists of two components,the first is independent of enthalpy change TδSâ—¦ and the second is proportional to αδHâ—¦. The enthalpy-entropy compensation was also investigated with K+ bis-crown ethers complex [19]. The results show the existence of this phenomena with α= 1.03 and intercept of 4.6 andcorrelation coefficient 0.99. Our results and these previously published are shown in Table 2 [19,20]. The slope α is related to the degree of ligandsconformational change and desolvation are involved upon complexation in each case and the intercept (TδSâ—¦) to the extent of cations and ligand salvation. Thus the largest α obtained for bis-crown ether reveals that this class of ligand experience substantial conformational change.The second largest intercept (TδSâ—¦) also demonstrates that the cation accumulated in the cavity suffers extensive desolvation. Quantitively, the unit slope α=1 and the large intrinsic entropy gain (TδSâ—¦ =4.6) jointly that the enthalpic gain arising from cation binding is completely canceled by the entropic loss from bis-crown ethers conformational change required upon complexation.
Conclusion
The electron- donor macrocyclic compounds used in this study and the electron -acceptor fullerene C60 form charge transfer complexeswith 1:1 stoichiometry. The obtained Kc values were dependent on many factors including cavity size, types of heteroatoms and substituents onthe macrocycle. The thermodynamic study showed an enthalpy-entropy compensation effect.
Further studies on different macrocyclic compounds with C60 are under investigation to study thoroughly the factors which effect the complexation constant.
References
- Chandra S, Sharma S (2006) J Indian Chem Soc 83: 988.
- Chandra S, Pundir M (2008) Spectroscopic characterization of chromium(III), manganese(II) and nickel(II) complexes with a nitrogen donor tetradentate, 12-membered azamacrocyclic ligand. Spectrochim Acta A Mol Biomol Spectrosc 69: 1-7.
- Bhattacharya S, Sharma A, Nayak S, Chattopadhyay S, Mukherjee A (2001) Spectrophotometric and thermodynamic study of supramolecular complexes of [60]- and [70]fullerenes with a number of calix[n]arenes. J Chem Soc Perkin Trans 2: 2292-2297.
- Mizyed S, Ashram M, Miller D, Georghiou P (2001) Supramolecular complexation of [60]fullerene with hexahomotrioxacalix[3]naphthalenes: a new class of naphthalene-based calixarenes. J Chem Soc Perkin Trans 2 1916-1919.
- Bhattacharya S, Sharma A, Nayak S, Chattopadhyay S, Mukherjee A (2003) NMR Study of Complexation of Crown Ethers with [60]- and [70]Fullerenes. J Phys Chem B 107: 4213-4217.
- Wang Y (1992) Photophysical properties of fullerenes and fullerene/N,N-diethylaniline charge-transfer complexes. J Phys Chem 96: 764-767.
- Andrade SM, Costa SMB, Pansu R (2000) STRUCTURAL CHANGES IN W/O TRITON X-100/Cyclohexane-Hexanol/Water Microemulsions Probed by a Fluorescent Drug Piroxicam. J Colloid Interface Sci 226: 260-268.
- Takahasi K, Horino K, Komura T, Murata K (1993) Photovoltaic Properties of Porphyrin Thin Films Mixed with o-Chloranil. Bull Chem Soc Jpn 66: 733-738.
- Eychmuller A, Rogach AL (2000) Chemistry and photophysics of thiol-stabilized II-VI semiconductor nanocrystals*. Pure Appl Chem 72: 179-188.
- Saha A, Nayak S, Chattopadhyay S, Mukharjee A (2003) Spectrophotometric Study of Complexation of Dicyclohexano-24-crown-8 with [60]- and [70]Fullerenes and Other Acceptors. J Phys Chem B 107: 11889-11892.
- Qarqaz E (2006) Charge Transfer Complexation between [60] Fullerene and Some Crown Ethers, M.Sc. Thesis, Yarmouk University.
- Al-Khalyfeh K (2012) A Thermodynamic Study of the Charge Transfer Complexes of C60 with Some Thiacrown Ethers, M.Sc. Thesis, Mu'tah University.
- Liu Y, Han J, Zhao Y, Zhang H, Yu Z (2005) Synthesis of Some Selenacrown Ethers and the Thermodynamic Origin of Their Complexation with C60. J Incl Phenom 51: 191-198.
- Atwood JL, Barbour LJ, Nichols PJ, Raston CL, Sandoval CA (1999) Symmetry-Aligned Supramolecular Encapsulation of C60: [C60⊂(L)2], L=p-Benzylcalix[5]arene or p-Benzylhexahomooxacalix[3]arene. Chem Eur J 5: 990-996.
- Al-Said N, Mizyed S, Al-Sehemi A, Kleeb A (2014) Naphthaldehyde-derived ligands: synthesis and their Ni(II) ion complexation. Monatsh Chem 145:275-279.
- Naghnaghia EB (2012) Synthesis and Characterization of New Schiff Base Crown Ethers Derived Salicylaldehyde and their Complexation, M. Sc. Thesis, Yarmouk University.
- Mizyed S, Kiwan R, Marji D (2013) Synthesis of New Azacrown Ether Schiff-Bases and their Complexes with C60. Jordan Journal of Chemistry 8: 71-78.
- Mizyed S, Al-Jarrah E, Marji D, Ashram M (2007) A spectrophotometric study of the charge transfer complexesof [60]fullerene with different tert-butylcalix[4]crowns. Spectrochim Acta Part A 68: 1274-1277.
- Liu Y, Tong L, Huang S, Tian B, Inoue Y, Hakushi T (1990) Complexation thermodynamics of bis(crown ether)s. 4. Calorimetric titration of intramolecular sandwich complexation of thallium and sodium ions with bis(15-crown-5)s and bis(12-crown-4)s: enthalpy-entropy compensation. J Phys Chem 94: 2666-2670.
- Inoue Y, Hakushi T (1985) Enthalpy-entropy compensation in complexation of cations with crown ethers and related ligands. J Chem Soc Perkin Trans 2 935-946.