Research Article
A Prospective, Open-label, Dietary Intervention to assess the Efficacy, Tolerability and Safety of Celnutra RDM in Remission of Type-2 Diabetes Mellitus in Indian Population
Mondal SK1, Pakhale KL2, Kothari MC3, Mashal AG4, Kashiva RY5, Samant B6, Barbhai MS7, Mahajan M8, Shah VG9, Gada P10, Sikhwal C11, Vyas N12, Pawar A13, Anam Golandaz A14, Deepika VK15, Joshi M16, Shetty Z17, and Nidhi Singh N18
1Senior Dietician, R.K.M.S.P, Kolkata, India.
2Metabolic Physician, Metabol, Mumbai, India.
3Diabetologist and General Physician, Metabol, Mumbai. India.
4Diet Consultant, Metabol, Mumbai, India.
5Director Medicine & Medical Services, HOD - Diabetes & Obesity, Noble hospital, Pune, India.
6Chief dietician, Kokilaben Hospital, Mumbai. India.
7Consultant Clinical Dietitian, Dr.Chonkar’s Chaitanya Heart Clinic, Mumbai, India.
8Clinical Dietitian, Dr.Chonkar’s Chaitanya Heart Clinic, Mumbai. India.
9Clinical Dietician and Diabetes educator, Gut healings, Mumbai, India.
10Senior Dietitian, Vagad medical Center, Mumbai, India.
11Shree Bhagwan Mahaveer Multispeciality, India.
12Clinical Nutritionist, Highland Superspeciality hospital, Mumbai, India.
13Consultant Clinical Nutritionist, Noble hospital, Pune, India.
14Senior Clinical Dietician, Head and Neck Cancer Institute of India, Mumbai
15Senior Clinical Dietitian, Connect and Heal Primary Care Pvt. Ltd, India.
16Senior Clinical Dietitian, Food N U, Nashik, India.
17Clinical Dietician and Diabetes educator, Fortis Hospital, Mumbai, India.
18Clinical Dietitian, Oncoheal, Pune, India.
2Metabolic Physician, Metabol, Mumbai, India.
3Diabetologist and General Physician, Metabol, Mumbai. India.
4Diet Consultant, Metabol, Mumbai, India.
5Director Medicine & Medical Services, HOD - Diabetes & Obesity, Noble hospital, Pune, India.
6Chief dietician, Kokilaben Hospital, Mumbai. India.
7Consultant Clinical Dietitian, Dr.Chonkar’s Chaitanya Heart Clinic, Mumbai, India.
8Clinical Dietitian, Dr.Chonkar’s Chaitanya Heart Clinic, Mumbai. India.
9Clinical Dietician and Diabetes educator, Gut healings, Mumbai, India.
10Senior Dietitian, Vagad medical Center, Mumbai, India.
11Shree Bhagwan Mahaveer Multispeciality, India.
12Clinical Nutritionist, Highland Superspeciality hospital, Mumbai, India.
13Consultant Clinical Nutritionist, Noble hospital, Pune, India.
14Senior Clinical Dietician, Head and Neck Cancer Institute of India, Mumbai
15Senior Clinical Dietitian, Connect and Heal Primary Care Pvt. Ltd, India.
16Senior Clinical Dietitian, Food N U, Nashik, India.
17Clinical Dietician and Diabetes educator, Fortis Hospital, Mumbai, India.
18Clinical Dietitian, Oncoheal, Pune, India.
*Corresponding author:Singh N, Department of Clinical Dietitian, Oncoheal, Pune, Maharastra, India. E-mail Id:
nidhisingh295@gmail.com
Article Information:Submission: 07/02/2025; Accepted: 28/02/2025; Published: 04/03/2025
Copyright: ©2025 Mondal SK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background:Traditional management of Type 2 diabetes emphasizes glycemic control through education and medication, assuming the condition is irreversible. However, it often fails to provide adequate control, highlighting the need for alternative approaches. Lifestyle therapies, including those targeting weight loss and Type 2 diabetes remission, are being explored, with oral nutritional supplements high in protein and low in carbohydrates showing particular promise. The aim was to evaluate the efficacy and safety of Celnutra RDM dietary supplementation in individuals with prediabetes and Type-2 diabetes.
Methods:This was a prospective, open-label, dietary intervention conducted in newly diagnosed participants with prediabetes or Type-2 diabetes. Participants were placed on Celnutra RDM dietary supplementation of 50 g once a day for 90 days. Anthropometric measurements, glycemic profiles, blood pressure and dietary intake were measured at baseline, day-45 and day-90 post intervention. Global overall patient rating responses on the efficacy and safety was obtained post-intervention.
Results:Celnutra RDM supplementation for 90 days resulted in a significant reduction in anthropometric, glycemic profiles and blood pressure. The nutritional supplementation significantly reduced median values of weight, BMI, waist circumference, and waist hip ratio from baseline to post-intervention timepoints (P<0.001). Similarly, the nutritional supplementation significantly reduced median fasting blood glucose, post-prandial blood glucose, and Hb1Ac levels from baseline to post-intervention (P<0.001). Majority participants rated the efficacy and safety profile of the supplementation as good to excellent.
Conclusion:Overall, Celnutra RDM appears to be a viable option for satisfying the unmet need for effective, safe, and well-tolerated nutritional therapy for weight reduction and diabetes remission in prediabetes and Type-2 diabetes.
Methods:This was a prospective, open-label, dietary intervention conducted in newly diagnosed participants with prediabetes or Type-2 diabetes. Participants were placed on Celnutra RDM dietary supplementation of 50 g once a day for 90 days. Anthropometric measurements, glycemic profiles, blood pressure and dietary intake were measured at baseline, day-45 and day-90 post intervention. Global overall patient rating responses on the efficacy and safety was obtained post-intervention.
Results:Celnutra RDM supplementation for 90 days resulted in a significant reduction in anthropometric, glycemic profiles and blood pressure. The nutritional supplementation significantly reduced median values of weight, BMI, waist circumference, and waist hip ratio from baseline to post-intervention timepoints (P<0.001). Similarly, the nutritional supplementation significantly reduced median fasting blood glucose, post-prandial blood glucose, and Hb1Ac levels from baseline to post-intervention (P<0.001). Majority participants rated the efficacy and safety profile of the supplementation as good to excellent.
Conclusion:Overall, Celnutra RDM appears to be a viable option for satisfying the unmet need for effective, safe, and well-tolerated nutritional therapy for weight reduction and diabetes remission in prediabetes and Type-2 diabetes.
Keywords:Nutrition; Dietary; Glycemic; Weight loss; Celnutra; Prediabetes; Type-2 diabetes
Introduction
The global prevalence of Type-2 diabetes is rising. The World
Health Organization (WHO) and International Diabetes Federation
(IDF) report that there are over 537 million diabetics globally.
Given the high frequency of prediabetes, this statistic is anticipated
to increase considerably in the next decades. In India, around 74
million individuals have diabetes, with one in every two adults going
untreated [1]. According to the most recent ICMR-INDIAB-13
research, 63.7% of individuals with diabetes had poor blood glucose
control, which is a major problem in India [2].
Classical type 2 diabetes management, based on the premise that
Type-2 diabetes is an irreversible and progressing chronic condition,
has concentrated on glycemic control through diabetes education
and increasing glucose-lowering medication usage
[3,4,5]. However,
this strategy does not result in appropriate glycemic control in a
substantial number of patients [3,4,5]. Obesity and excessive fat
accumulation in the liver and pancreas are strongly associated with
Type-2 diabetes [3,6,7]. Studies, including the Diabetes Remission
Clinical Trial (DiRECT), Counterpoint, and Counterbalance, have
challenged this initial result and documented that effective diet induced
weight loss has been shown to result in long-term Type-2
diabetes remission [8,9,10,11,12]. Thus, weight management is
rapidly taking priority in Type-2 diabetes, with substantial evidence
that remission may be accomplished with weight loss interventions.
The American Diabetes Association (ADA) defines remission
as HbA1c <6.5% (48 mmol/mol) measured at least 3 months after
cessation of glucose-lowering pharmacotherapy, which is the
standard diagnostic criteria [13].
Recently, the emphasis has switched toward intensive lifestyle
interventions to achieve weight reduction and diabetes remission.
Oral nutritional supplement therapy are promising options, which
involve high protein and low carbohydrate dietary interventions to
accomplish weight loss and Type-2 diabetes remission
[14,15,16].
Celnutra RDM is a nutritionally balanced supplement designed
for sustained energy release. It features a high-protein, low-glycemic
formulation with low carbohydrate content, which has the potential
for weight reduction management and diabetes remission in Type-2
diabetes. Therefore, this study was conducted to evaluate the efficacy
and safety of Celnutra RDM dietary supplementation in individuals
with prediabetes and Type-2 diabetes.
The primary objectives of this study were to evaluate the
changes in the anthropometric, glycemic profiles and blood pressure
after Celnutra RDM dietary supplementation in individuals with
prediabetes and Type-2 diabetes.
Methods
Study design:
This was a prospective, open-label, clinical study conducted at
Multiple hospitals across India.Ethical Consideration:
Ethical committee approval was obtained from the participating
hospital. The study was carried out in accordance with the
Declaration of Helsinki, ethical standards established by the Indian
Council of Medical Research (ICMR), and good clinical practice
(GCP). Informed written consent was taken from the participants.Study participants:
The study participants included newly diagnosed prediabetic and
Type-2 diabetic patients.Sample size:
It was a convenient sample size. A total of 149 participants
attending the hospital sites during the study period were enrolled.Participant eligibility criteria:
Newly diagnosed participants of either gender with more than
25 years with prediabetes or Type-2 diabetes according on American
Diabetes Association guidelines, with fasting blood glucose ≥100
mg/dL for prediabetes or ≥126 mg/dL for Type-2 diabetes, and/or
glycated haemoglobin (HbA1c) ≥5.7% for prediabetes or ≥6.5% for
Type-2 diabetes, not consuming antidiabetic drugs were included.Exclusion criteria for the study included newly diagnosed Type-2
diabetes with HbA1c levels ≥9%, Type-2 diabetes on hypoglycemic
drugs, regular intake of nutraceuticals products or multivitamin
supplement (last 3 months) that may affect lipid and/or glycemic
metabolism, currently on weight loss medications or (last 3 months)
or , and any other severe comorbid condition that could impact
study outcomes, known hypersensitivity to the contents of the
interventional nutraceutical product, participants not willing for
dietary intervention compliance and not willing for written consent.
Interventional dietary supplementation/nutraceutical product:
The proposed nutraceutical/ dietary intervention supplement
was CELNUTRA RDM, a high-protein supplement with low
glycemic and carbohydrate content. It is an exclusive product of
NUCGNEX LIFESCIENCES, India[17]. The product contains whey
proteins, milk solids, micronutrients such as vitamins and minerals,
as well as various functional ingredients[17]. Participants were given
proposed nutritional supplement product containing a net 400 g of
the supplement. Participants were advised to take the intervention
supplement in a quantity of 50 g (approximately 5 leveled scoops) in
170 ml water once a day for 90 days. One higher-calorie meal each
day was substituted by the proposed nutraceutical supplement.Study procedure:
Written Informed consent was obtained from the participants
during screening and enrolment. The participants were screened
for brief medical history, general physical examination, vital
parameters and nutritional assessment was conducted by a certified
qualified dietician. Once enrolled, the participants were assessed
for the efficacy parameters: anthropometric parameters, glycemic
parameters and blood pressure. For 90 days, participants received
50 g of Celnutra RDM as a dietary supplement replacing one meal
of the day. Anthropometric, glycemic, and blood pressure baselines
were measured and evaluated on Day-45 and Day-90 after the dietary
intervention. Post-intervention, global overall patient ratings of
effectiveness and safety were obtained from the participants.Primary outcome:
EfficacyChange in the anthropometric parameters, which included weight, waist circumference, hip circumference and waist to hip ratio.
Change in the glycemic parameters, which included fasting blood glucose, post prandial blood pressure and HbA1c levels
Change in the systolic and diastolic pressure parameters
Safety assessment
Assessment of the adverse events reported was considered as part of the safety evaluation
Secondary outcomes:
Patient rated Global Assessment of Efficacy and Safety of the
supplementPatient-rated global evaluation scales were utilized to determine clinically relevant two elements of treatment outcome: a perceived efficacy and tolerability /safety of the proposed nutraceutical supplement.[18] It consisted of a four-category ordinal response scale for the participants (‘Poor’, ‘Fair’, ‘Good’ and ‘Excellent’) for assessment.
Statistical Analysis:
Statistical Package for the Social Sciences (IBM SPSS) Statistics
for Windows, Version 21 was used for the statistical analysis.
Descriptive statistics were used to express the data in terms of
actual numbers, percentage, mean with standard deviation (SD) and
median with interquartile range (IQR). Data normality was assessed.
Nonparametric test Friedman Test and Wilcoxon Signed Ranks Test
were used. A P-value of <0.05 was considered statistically significant.Results
Baseline characteristics:
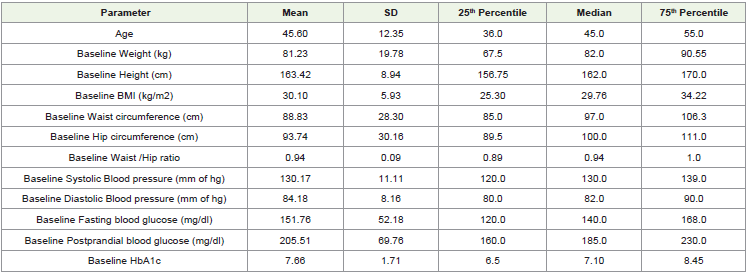
A total of 149 diabetics participated in this study. The mean age of
the patients was 45.58 ± 12.39 years with 49% males and 51% females.
The male to female ratio was 0.96. The baseline characteristics of the
patients are summarized in [Table 1].Pre/post intervention Outcomes:
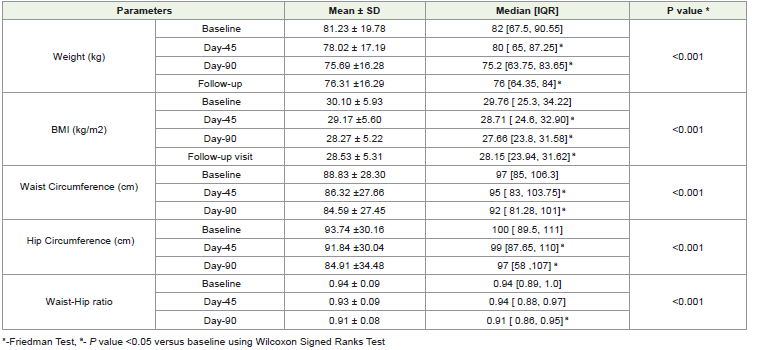
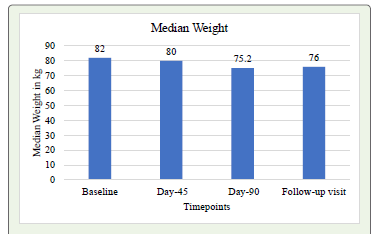
Changes in Antropometric parametersThe intervention led to significant reduction in the median weight from baseline of 82 kg to 80 kg at Day-45, further decreasing to 75.2kg at Day-90 (P<0.001). At follow-up visit, the median weight was 76 kg, indicating significant sustained loss (P<0.001). [Graph 1] The post hoc analysis of baseline median weight compared to weight at different time points (Day-45, Day-90 and Follow-up visit) showed significant reductions in median weight post -intervention (P<0.001). Similarly, it was observed that, the intervention led to significant reduction in median values of anthropometric parameters like the BMI, waist circumference and waist hip ratio from baseline to post intervention time points (P<0.001). [Table 2]
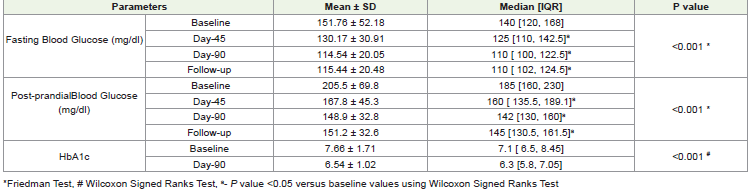
Changes in Glycemic profile:
The intervention significantly reduced median fasting glucose
from a baseline of 151.76 mg/dL to 130.17 mg/dL at Day-45 and further
to 114.54 mg/dL at Day-90 (P<0.001). Similarly, the intervention
also significantly reduced median postprandial blood glucose from
baseline of 205.5 mg/dL to 167.8 mg/dL at Day-45 and 148.9 mg/dL
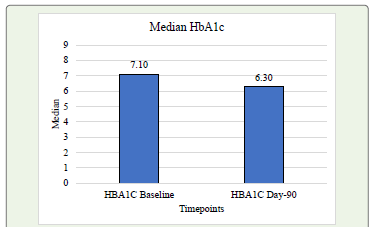
at Day-90 (P<0.001). [Table 3] The HbA1c level showed a significant
decrease from baseline of 7.66% to 6.54% post-intervention Day-90.
[Graph 2] These findings suggests that the intervention had a positive
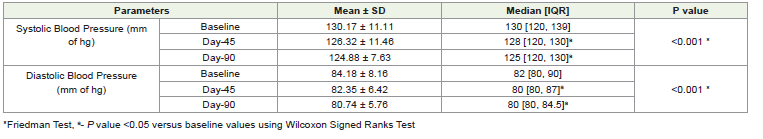
impact on glycemic profileChanges in Blood pressure:
The intervention significantly reduced the median systolic blood
pressure from a baseline of 130 mm of Hg to 128 mm of Hg at Day45 and further to 125 mm of Hg at Day-90 (P<0.001). Similarly,
the intervention significantly reduced median diastolic blood
pressure from a baseline of 82 mm of Hg to 80 mm of Hg at Day-
45 and further to 80 mm of Hg at Day-90 (P<0.001). [Table 4] This
continuous decline indicates effective management of blood pressure
over timepoints.
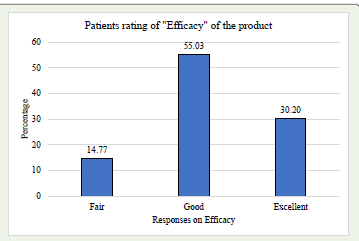
Patient rated – outcomes:
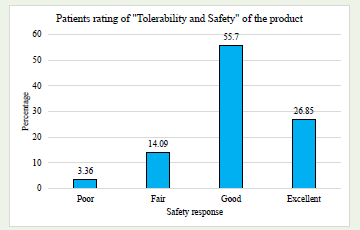
Patient rated- EfficacyA majority of patients rated the efficacy of product as “Good” (55.03%) and “Excellent” (30.2%). [Graph 3] Similarly, majority rated safety and tolerability of the product as “Good” (55.7%) and “Excellent” (26.85%), [Graph 4] indicating a generally positive view of the treatment’s efficacy and safety profile.
Adverse events:
In a total of 149 patients, 11 experienced adverse events (AEs)
of Gastro-intestinal tract (abdominal bloating and loose stool),
representing 7.38% of the cohort. Notably, there were no serious
adverse events (SAEs) observed in the cohort.Discussion
This study found that supplementation of Celnutra RDM, a high
protein, low glycemic and carbohydrate dietary supplement, daily for
90 days resulted in a substantial decrease (p < 0.001) in anthropometric
parameters, including weight, as well as improvement in glycemic
profiles and blood pressure in newly diagnosed prediabetics and
Type-2 diabetics.
Type-2 diabetes is still a devastating medical condition with a high
global prevalence. Obesity and increased fat accumulation in the liver
and pancreas are strongly linked to Type-2 diabetes [3,4,5,6]. Weight
management, in contrast to intensified medication therapy and
glycemic control, are rapidly taking center stage in Type-2 diabetes
[9,10,12] The focus has recently switched to intensive lifestyle
interventions which involve high protein and low carbohydrate
dietary interventions to accomplish weight loss and Type-2 diabetes
remission.
High-protein diets offers myriad health benefits and offers to
manage blood glucose levels in Type-2 diabetes. It aids in decreasing
glucose absorption, enhancing insulin sensitivity, increasing satiety,
improvement in lipid profile and immunity, and retaining muscle
mass, and reducing the glycemic impact of meals helping in weight
management [19]. Low glycemic index contents give continuous
energy throughout the day and prevent rapid glucose spikes, resulting
in more stable blood glucose levels[20,21]. These combined effects
contribute to better blood glucose control and help in managing or
preventing complications associated with diabetes.
In the present study, we explored the efficacy, tolerability and
safety of Celnutra RDM, a high-protein, low-glycemic formulation
in newly diagnosed individuals of prediabetes and Type-2 diabetes.
In the present study, daily consumption of Celnutra RDM for 90
days resulted in a significant reduction in anthropometric parameters.
The nutritional intervention significantly reduced median values of
weight, BMI, waist circumference, and waist hip ratio from baseline
to post-intervention timepoints (P<0.001), making it a promising
option for weight control, an important parameter for metabolic
health. Similarly, the nutritional intervention significantly reduced
median fasting blood glucose, post-prandial blood glucose, and
Hb1Ac levels from baseline to post-intervention (P<0.001). The
decrease in HbA1c levels is critical, since it is one of important
indicator of diabetes remission.
Celnutra RDM is a nutritionally balanced supplement designed
for sustained energy release. It features a high-protein, low-glycemic
formulation with low carbohydrate content, enriched with essential
vitamins and minerals. Key components include [17]:
•Low Carbohydrate Blend: A combination of maltodextrin and
glycerin with a low glycemic index, free from sucrose.
•High in Fiber: Coated with a blend of cellular carbs such as
Nutriose FB 06, hydrolyzed guar gum, and inulin, promoting early
satiety, reducing cravings, and enhancing gut health.
•Protein Blend: A slow-release protein formula comprising whey
protein and calcium caseinate, with a Protein Digestibility Corrected
Amino Acid Score (PDCASS) of 1.0, ensuring high protein quality.
•Probiotics: Includes Lactobacillus Rhamnosus, Bifidobacterium
Bifidum, Lactobacillus Acidophilus, And Bacillus Coagulans to
support gut dysbiosis and also exerts glycemic-moderating effects.
•Special nutrients: Enriched with Myoinositol, Arginine, Taurine,
Carnitine, And Coenzyme Q10, which contribute to the management
of diabetic complications by moderating glycemic levels and reducing
inflammation and oxidative stress.
•Functional ingredients: Contains Fenugreek extract, Gymnema
Sylvestre extract, and Glycyrrhiza Glabra extract, which aid in
regulating insulin sensitivity[17].
A balance combination of the components commensurate
provides weight loss, glycemic-moderating effect and promotes
remission.
In this study, it was also observed that nutritional intervention was well tolerated, only 7.38% experienced mild AEs of gastrointestinal tract (abdominal bloating and loose stool). No severe or serious adverse events were encountered during the study period.
Overall, Celnutra RDM appears to be a viable option for satisfying the unmet need for effective, safe, and well-tolerated nutritional therapy for weight reduction and diabetes remission in individuals with prediabetes and Type 2 diabetes.
In this study, it was also observed that nutritional intervention was well tolerated, only 7.38% experienced mild AEs of gastrointestinal tract (abdominal bloating and loose stool). No severe or serious adverse events were encountered during the study period.
Overall, Celnutra RDM appears to be a viable option for satisfying the unmet need for effective, safe, and well-tolerated nutritional therapy for weight reduction and diabetes remission in individuals with prediabetes and Type 2 diabetes.
Strength of the study:
The strength of this study lies in its provision of real-time evidence
across multiple hospitals regarding a nutritional formulation that is
high in protein, low in glycemic index, and low in carbohydrates,
while being enriched with essential vitamins, minerals, probiotics,
and functional ingredients. This formulation has been shown to
reduce key anthropometric parameters, including body weight, as
well as improve glycemic parameters and blood pressure in newly
diagnosed prediabetes and Type-2 diabetes individuals. The findings
of this study are particularly valuable for the broader application of
dietary management strategies in the treatment of diabetes.We are presently working on long-term monitoring of these individuals in order to determine the dietary intervention’s long-term effectiveness. We want to publish this extended data in the future, adding new insights to the field of diabetes management.
Limitation of the study:
The study was conducted with a modest sample size. Larger
population studies with a broader cross-section of individuals and
multiple locations may yield more conclusive results. Second, the
study looked at the mid-term effects of the dietary supplement on
anthropometric and glycemic profiles. Confirming the findings
in longer-term research might assist to better formulate dietary
recommendations for prediabetes and type 2 diabetes prevention and
management. Third, there was no comparator or control arm since
the research design was a quasi-experiment in a real-world setting.Conclusion
Celnutra RDM appears to be a viable option for satisfying
the unmet need for effective, safe, and well-tolerated nutritional
therapy for weight reduction and diabetes remission in individuals
with prediabetes and type 2 diabetes. The findings of this study
are particularly valuable for the broader application of dietary
management strategies for prediabetes and Type-2 diabetes.