Research Article
A Study of Autologous Serum Skin Test and Autologous Serum Therapy in Chronic Urticaria
Chandarana VT*, Bhuptani NV, Patel BK and Raghavon UN
Department of Dermatology, Venerology and Leprosy, PDU Government Medical College and Hospital, Rajkot, Gujarat, India
*Corresponding author: Chandarana VT, Department of Dermatology, Venerology and Leprosy, PDU
Government Medical College and Hospital, Rajkot, Gujarat, India, E-mail: chandaranavanashri@gmail.com
Copyright: © 2022 Chandarana VT, et al. This is an open access article distributed under the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original
work is properly cited.
Article Information: Submission: 04/01/2022; Accepted: 07/02/2022; Published: 10/02/2022
Abstract
Background: Chronic urticaria is defined as the recurrence of short-lived wheals three or more times per week for more than six weeks, with or without
angioedema. Chronic urticaria is a frustrating condition that affects at least 0.1 percent of the population. Patients with chronic urticaria suffer from irritated itch
and wheals, as well as a high antihistamine tablet load. The majority of chronic urticaria sufferers have an unknown or idiopathic cause. A subset of patients of
chronic urticaria may have an autoimmune basis for their condition. The objective of the study was to compare the effectiveness of AST in patients with ASST
positive and negative ASST and to see how AST affects the dermatological life quality index (DLQI) before and after treatment.
Methods: An interventional study was conducted in the Department of Dermatology OPD of our institute from October 2020 to October 2021. Thirty-Five
patients were included in our study. The ASST of 35 patients included routine and specialized laboratory testing (thyroid function test, stool for ova). Patients
were instructed to stop using antihistamines two days before the test. All patients had ASST conducted after receiving written authorization, and AST was
administered to them regardless of their ASST status (2ml autologous serum i.m. in gluteal region once weekly for 9 consecutive weeks). Patients in both
groups were told to take one tablet of levocetrizine (10 mg) if they felt wheals or itching, but not more than one pill per day. After 9 AST injections, a 4-week
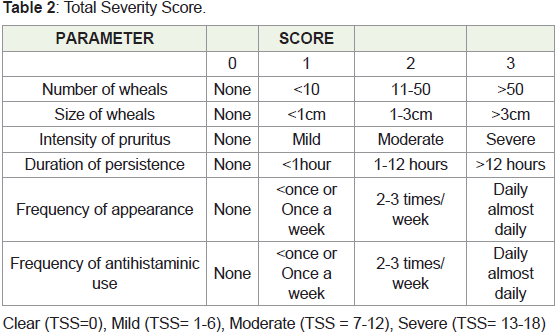
follow-up was taken. The key efficacy criteria were the urticaria activity score (UAS), total severity score (TSS), and DLQI, which were measured at baseline
and weekly after each injection of autologous serum treatment.
Results: In patients of both groups, UAS and TSS showed significant improvement (>50%) after 5th week of therapy. In ASST positive patients, the
improvement in DLQI score was slightly higher. A larger percentage of ASST positive patients enjoy symptom-free periods after a one-month follow-up period.
Conclusions: Both ASST positive and ASST negative individuals showed improvement in their symptoms. Patients with ASST positivity, on the other
hand, showed a greater increase in quality of life as measured by the length of time they were symptom-free.
Keywords
Autologous serum skin test; Autologous serum therapy; Autoimmune urticaria; Urticaria activity score; Total severity score; Dermatologic life
quality index
Introduction
Chronic urticaria is defined as the development of cutaneous
wheals that occur on a regular basis (usually daily) for >6 weeks with
individual lesions lasting from 4 to 36 hours [1].
Urticaria comes from the Latin term “urtica,” which refers to the
stinging nettle plant, which is now discovered to contain histamine.
The majority of chronic urticaria sufferers have an unknown or idiopathic cause. Chronic idiopathic urticaria or, more recently,
chronic spontaneous urticaria are terms used to describe this
condition. Autoimmune chronic urticaria is characterized by the
presence of circulating IgG autoantibodies directed against the high
affinity IgE receptor FcR1 on cutaneous mast cells, basophils, or, less
typically, IgE itself [2
].
ASST is used to identify autoimmune chronic urticaria.
Debbarman et al. found autologous serum therapy (AST) to be a promising approach for urticaria treatment, independent of ASST
status (ASST positive or ASST negative) [3
].
Chronic urticaria is a frustrating condition that affects at least 0.1
percent of the population. Patients with chronic urticaria suffer from
irritated itch and wheals, as well as a high antihistamine tablet load
[3]. As a result, newer effective modalities that reduce pill burden are
required.
Aims and Objectives
• To compare the effectiveness of AST in patients with ASST
positive and negative ASST.
• To see how AST affects DLQI before and after treatment.
Methods
An interventional study was conducted in the Department of
Dermatology OPD of our institute from October 2020 to October
2021. Thirty-Five patients were included in our study.
Inclusion Criteria:
We have included all patients above 18 years of age with refractory
chronic urticaria in our study.Exclusion Criteria:
• Chronic urticaria due to predominantly physical causes
• Pregnancy and lactation
• Severe systemic illness
• Anticoagulation therapy
• Corticosteroids or immunosuppressive therapyScoring system used:
• Urticaria Activity Score (UAS)
• Total Severity Score (TSS)
• DLQI
• Other scoring systems which can be used include• Angioedema Activity Score(AAS)- Recommended for
urticaria as standard measurement for assessing disease
activity in patients with recurrent angioedema (RAE)
• Chronic Urticaria Quality of Life(CU-Q2oL) – Developed
to refer to the disease’s impact on and therapy in a patient’s
life, according to his/her perception
• Angioedema Quality of Life(AE-QoL) – It is a specific patient
related outcome tool to assess quality of life impairment in
recurrent angioedema patients
• Urticaria Control Test (UCT) - It is the first valid and reliable
tool to assess disease control in patients with chronic urticaria.
It is a retrospective approach and simple scoring system
• Urticaria Severity Score(USS) – It includes 12 questions and
7 responses per questions
• UAS - i.e. UAS calculated for 7 days once daily or twice daily
[7]
Here, in our study we have used UAS scoring system for follow up
due to following advantages
• Easy to calculate score
• Less time consuming
• Once weekly monitoring required
• Patients’ opinion about their symptoms like intensity of
pruritus can be taken into consideration
• Every week by calculating UAS we can know if patient is
improving or not after starting therapy
• Psychologically it gives sense of relief to patients on
improvement of their score
However, it has certain limitations like interindividual variations
are present, variations in perception of symptoms like pruritus by
patients, different number of wheals at different times of the day and
also on different days, difficulty or error in calculating total number
of wheals.
In an organised proforma, we have documented a complete
history and examination. The ASST of 35 patients included routine
and specialised laboratory testing (thyroid function test, stool for
ova). Patients were instructed to stop using antihistamines two days
before the test. All patients had ASST conducted after receiving
written authorization. Autologous serum is prepared by collecting
5ml of patient’s venous blood in a sterile vacutainer from which serum
is separated by centrifugation at 2000X for 10 minutes. The serum
separated by centrifugation is used immediately for ASST. Normal
saline was used as a control. Approximately 0.05ml of autologous
serum and normal saline is injected separately intradermally over
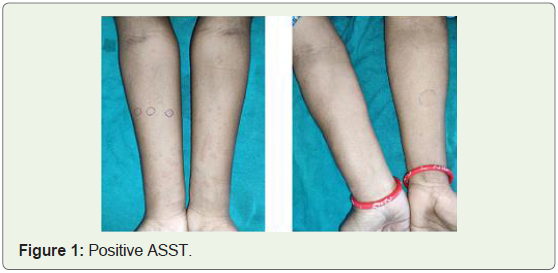
volar aspect of forearm. Positive ASST is one with serum induced
wheal which has a diameter of >= 1.5mm as compared to saline
induced wheal at 30 minutes.AST was administered to patients
regardless of their ASST status (2ml autologous serum i.m. in gluteal
region once weekly for 9 consecutive weeks). Patients in both groups
were told to take one tablet of levocetrizine (10 mg) if they felt wheals
or itching, but not more than one pill per day. After 9 AST injections,
a 4-week follow-up was taken. The key efficacy criteria were the
urticaria activity score, total severity score, and dermatologic life
quality index, which were measured at baseline and weekly after each
injection of autologous serum treatment. When the average of two
perpendicular diameters of the wheal was 1.5 mm greater than the
saline wheal, ASST was termed positive.
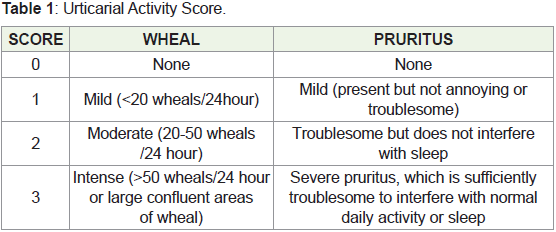
Urticarial activity score which measure two symptoms, number of
wheals (0-3 scale per day) and intensity of pruritus (0-3 scale per day)
is given in Table 1 [4].
Urticaria Activity Score = Wheal score + Pruritus score
Dermatology life quality index:
A validated vernacular (Gujarati) version of the dermatological
life quality index (http://www.dermatology.org.uk/downloads/DLQI Gujarati.pdf) was used to measure quality of life in urticaria patients.
The DLQI consists of 10 items, each of which is rated between 0 and
3. Following the AST, each patient was scored to see how their quality
of life (QoL) improved after treatment.Results
A total of 35 instances were investigated. There were 19 females
and 16 males in the group. The majority of the patients, 21 (60
percent), were between the ages of 20 and 40, with a range of 18 to
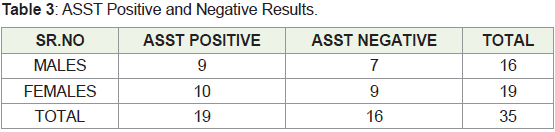
65 years. One patient (2.86 percent) had a family history. ASST was
positive in 19 patients (54.29 percent) in group A and negative in 16
patients (45.7 percent) in group B as shown in Table 3. (7/19)36.84
percent of group A patients and (3/16)18.8 percent of group B patients
had a history indicative of atopy. Thyroid function tests were positive
in 7/19 individuals (36.84%) in group A and none in group B. After 9
weeks of therapy, we took a four-week follow-up and found that 73.68
percent of Group A patients and 62.5 percent of Group B patients
were still entirely symptom-free from urticaria symptoms.
Discussion
Urticaria has been proved to have a major influence on patients’ quality of life in the areas of mood, functionality, and symptoms since
the time of Heberden, who first reported it. Chronic urticaria has an
uncertain course, and therapy is continued until the condition is in
remission. The need for innovative treatment modalities to support
antihistamines and leukotriene inhibitors has long been recognised,
and any adjuvant therapy that might minimise pill load while attaining
symptom-free periods is urgently required. The improvement in
quality of life (as judged by the DLQI) was shown to be considerable
in individuals receiving AST in our study. The objective of treatment
for chronic urticaria is to keep patients symptom-free for as long as
possible while minimising side effects. The impact of methodology
differences, the difficulty of precisely identifying a positive response,
and the interpretation of data are only a few of the challenges in
characterising ASST positivity. As a result, fresh strategies are still
necessary for ASST standardization. The percentage of ASST positive
patients in our research (54.29%) was greater than that reported
by Vikramkumar et al. (41.6%) [6]. Thyroid function tests were
positive in seven individuals (36.84 percent of Group A patients and
none of Group B patients), indicating a link between thyroid auto
antibodies and auto reactive urticaria, as reported by George et al [7].
Group A patients had a higher baseline mean UAS (4.36 +/- 0.955)
and TSS (13.47+/- 2.116) than Group B patients, who had a lower
baseline mean UAS (3+/- 0.63) and TSS (11.375+/-1.204), which was
statistically significant in comparison to George et al study, which
found a trend toward a significant association between the severity of
chronic urticaria and ASST positivity.7 However, not all studies have
found a significant difference in UAS or TSS between ASST-positive
and ASST-negative individuals, indicating that these patients’ UAS
and TSS are varied. After 5 weeks of therapy, both groups of patients
showed a more than 50% improvement in UAS. The improvement in
UAS and TSS was greater in Group A participants after 9 weeks of
treatment. In Group A patients, the improvement in DLQI score was
also higher. A larger percentage of Group A patients enjoy symptom-free
periods after a one-month follow-up period.
Autologous Serum Therapy demonstrated to be an adjuvant
therapy in our research of ASST positive urticaria patients who were
otherwise unresponsive to conventional therapy.
Conclusion
Patients with ASST positive had more severe urticaria symptoms.
Both ASST positive and ASST negative individuals showed
improvement in their symptoms. Patients with ASST positivity, on the
other hand, showed a greater increase in quality of life as measured by
the length of time they were symptom-free (Figure 1&2).