Review Article
Prevalence of Genetic Variations AffectingWarfarin Action from Different Parts of India
Kavita Shalia1*, Sunila Raju1 and Amit Chandan2
Corresponding author: Kavita K. Shalia, Sir H.N. Medical Research Society, Sir H.N. Hospital and Research Centre,Court House, L. T. Road, Mumbai 400 002, India, E-mail: Kavita.Shalia@rfhospital.org
Citation: Kavita Shalia. Prevalence of Genetic Variations Affecting Warfarin Action from Different Parts of India Indian J Cardio Biol Clin Sci. 2016; 3(1): 108.
Copyright © 2016 Kavita Shalia, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Indian Journal of Cardio Biology & Clinical Sciences | Volume: 3, Issue: 1
Submission: 27/10/2016; Accepted: 21/12/2016; Published: 23/12/2016
Abstract
Warfarin, an anticoagulant is used in patients who are at increased risk of developing blood clots. The management of warfarintherapy is challenging because it shows large inter-individual variability in patient ’s response. Studies implicate that variability in warfarin dosage, among several other factors, is largely determined genetically. Genetic factors for warfarin mainly involve polymorphisms ofVitamin K epoxide Reductase Complex (VKORC) subunit 1 (VKORC1) and cytochrome P-450 enzyme 2C9 (CYP2C9) enzymes. There is general agreement among published retrospective population studies that the combination of VKORC1 and CYP2C9 genotypes, together with gender, age, body mass index or height or weight and concurrent medications, predict approximately 50% of warfarin dose requirement. Thus, genetic testing will be able to predict the correct dose and reduce trial and error as well as risks during therapy. Within the Asian population, Indian patients have been reported to require higher warfarin dose than others. Here we review the prevalence of the genetic variations of VKORC1 and CYP2C9 associated with variable warfarin response in Indian population.
Keywords: VKORC1; CYP2C9; Single nucleotide polymorphism; Warfarin; Indian population
Introduction
The most frequently prescribed class of oral anticoagulants are the vitamin K antagonists (VKAs). Many of these agents are derivedfrom coumarin and include warfarin, dicoumarol, acenocoumarol and phenprocoumon. Warfarin is the most widely prescribed oral anticoagulant agent. The name ‘warfarin’ is derived from the group at the University of Wisconsin who discovered it, the Wisconsin Alumni Research Foundation (WARF), with the ending of ‘-arin’ indicating it’s link to coumarin. Coumarin is a chemical that is found in sweet clover hay. Coumarin does not affect the coagulation system but is converted to dicoumarol, which is a powerful anticoagulant, in spoiled animal feeds. This had led to the death of many cattle due to internal haemorrhage during a warm year in the 1920’s. Warfarin is a synthetic derivative of dicoumarol which was developed in 1948 as a rodenticide and in the 1950s was found to be effective in the prevention of thrombosis. It has been used as an anticoagulant in clinical practice since 1954.
It is the most widely prescribed oral anticoagulant agent in thromboembolic prophylaxis. However, therapy with this Vitamin K antagonist is challenging as it has a narrow therapeutic index andinter-individual variations, same fixed dose of warfarin for all patientshas been found to be impractical and inappropriate as it is not easilyadjustable. An insufficient dose may result in thrombosis, whereas overdose may lead to unexpected bleeding [1]. The daily maintenance dose for warfarin ranges from 0.5 to 80 mg2 [2].
Mechanism of Action
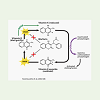
Warfarin is primarily metabolized in liver by cytochrome P450enzyme and gets completely absorbed after oral administration withpeak concentration generally attained within the first four hours. Itexerts its anti-coagulant effect by inhibiting the activity of VKORC1protein [Figure 1] [3]. It consists of a racemic mixture of two enantiomers, R- and S-warfarin, each of which is cleared by different pathways. The S-form is metabolized by the polymorphic cytochrome P-450 enzyme CYP2C9, whereas several enzymes including CYP1A2 and CYP3A4 clear the R-form. The half-life of R-warfarin is reported to range from 37 to 89 hours, while that of S-warfarin is reported to range from 21 to 43 hours [4]. S-isomer is three to five times more potent than the R-form [5].
Vitamin K epoxide reductase (VKOR) is an enzyme that reduces vitamin K after it has been oxidized in the carboxylation of glutamic acid residues in blood coagulation factors II, VII, IX and X. The antithrombotic effect of warfarin is achieved by hindering the activation of Vitamin K dependent clotting factors II, VII, IX and X. Its a ticoagulant effect is not seen instantly since it only inhibits theformation of new functioning coagulation factors, and has no effect on their elimination. Changes in anticoagulant effect, as measured by the prothrombin time (PT) are typically noted 24-36 hours after start of treatment and reflect the decrease in total activity of factors II, VII and X [6].
Monitoring of Warfarin Dose
The International Normalized Ratio (INR) [INR=(PTpatient/PTcontrols)ISI] of the plasma Prothrombin Time (PT) test is commonly adopted for laboratory monitoring of oral anticoagulant therapy and as a guide to Warfarin dose adjustment. This was proposed by the World Health Organization (WHO) in 1982. Before this time the PTwas used. PT is measured in seconds and the test is performed by adding calcium and thromboplastin to citrated plasma. It had some significant drawbacks, the main problem was lack of standardization caused by the use of different thromboplastins which vary in responsiveness to reduction in the vitamin-K dependent coagulation factors which led to different results when compared to different laboratories. INR is standardized by dividing the PT of a patient with the geometric mean of PT for at least 20 healthy subjects with the same test system and adjusting the result according to the international sensitivity index (ISI) of the thromboplastin used in the lab. As a result of the standardization, an INR value of 1.0 is considered to be normal coagulation [7]. INR of 2.5 to 3.5 is recommended in patient with mechanical prosthetic heart valves [8].
Adverse Effects
The clinical effectiveness of warfarin is established. However, because of a low therapeutic index, bleeding frequently complicates anticoagulation with warfarin. Overall, the bleeding rate is 7.6 to 16.5 per hundred patient/year. Major or life-threatening bleeds occur at a rate of 1.3 to 2.7 per hundred patients/year [9-11]. Although major bleeding can occur at therapeutic levels, the risk of bleeding rises with increasing intensity of anticoagulation [12]. Thus, the management of warfarin therapy is challenging due to large interindividual variability in patients response, which may be a result of various factors such as age, gender, diet, concurrent drug interaction and genetic factors. Identification of risk factors for the development of a high INR may identify patients at high risk of bleeding [13].
Factors Affecting Warfarin Dose
Warfarin dosage mainly depends on age, height, body surfacearea, environment, dietary, drug interactions, co-morbidities andgenetic factors. Dietary factors like dark green vegetables such asbroccoli, brussels sprouts and spinach contain large levels of vitaminK. It is estimated that an increase in vitamin K intake of 100 μg perday for 4 consecutive days lowers the INR by 0.2 [14]. Drugs like amiodarone, potentiates warfarin anticoagulation through inhibition of its metabolic clearance, rifampicin and carbamazepine in contrast increase its hepatic clearance. Drugs that inhibit clotting like aspirin, diclofenac and ibuprofen, having no inhibiting or inducing effect on warfarin are also regarded as interacting drugs since they increase the risk of bleeding [15].
Genetic Factors
Genetic factors for warfarin mainly involve polymorphismsof Vitamin K epoxide Reductase Complex (VKORC) subunit 1 (VKORC1) and CYP2C9 enzyme. Polymorphisms are genetic variations that occur in a population at a frequency of 1%. Among these, single nucleotide polymorphisms (SNPs) are the most common, wherein there is a change in a single base. Thus, variations in the gene sequence can affect level of its genetic and protein expression, resulting in inter-individual variation in the amount of protein or enzyme activity. Among our many happening metabolic processes, the action of drug is also dependent on these processes on enzymes, receptors etc. for its activation, action and final metabolism. This introduces the term “Pharmacogenetics†and “Pharmacogenomicsâ€
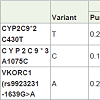
Cytochrome P-450 2C9 (CYP2C9): The CYP2C9 gene is located on chromosome 10 (10q242). Its gene product is the principle enzyme that terminates the anticoagulant effect of warfarin by catalyzingthe conversion of the pharmacologically more potent S-enantiomer to its inactive metabolites. CYP2C9 is highly polymorphic and more than 30 variants with altered catalytic properties have been identified [16]. The wild-type allele CYP2C9*1 is associated with normal enzyme activity and individuals who are homozygous for CYP2C9*1 have the normal, or “extensive metabolizerâ€, phenotype. In addition to the wild-type CYP2C9*1 allele, 2 allelic variants, 2C9*2 (C430T, rs1799853) causing R144C and 2C9*3 (A1075C, rs1057910) causing I359L, with approximately 12% and 5% enzymatic activity, respectively, have been identified [17-19]. These variations decreasethe degradation and clearance of warfarin and therefore, a low dosageis needed to maintain therapeutic INR [20]. Compared to normal metabolizers, patients who inherit one or two copies of *2 or *3 are more sensitive to warfarin - they require lower starting doses and are at a greater risk of bleeding during warfarin therapy [21-23]. Patients with variant alleles might be susceptible to over-anticoagulation or during a change of medication, particularly if an additional drug is competitively metabolized by the cytochrome P-450 system. The above two variants of the CYP2C9 gene contribute to about 10% of the dose variation of warfarin between patients. The frequencies of the CYP2C9 alleles vary between different ethnic groups. The *2 allele is more common in Caucasian (10-20%) than Asian (1-3%) or African (0-6%) populations [24]. The *3 allele in Asian countries (0.039) and Caucasian population (0.057) [25] and is less common (<10% in all populations) and extremely rare in African populations [26]. In African Americans, it is likely that other CYP2C9 variants such as CYP2C9*5, *6, *8, and *11 contribute to the variability in patient response to warfarin [27].
Vitamin K epoxide Reductase Complex subunit 1 (VKORC1): VKORC1 gene is located on chromosome 16. The biological activity of all known vitamin K-dependent blood clotting factors is highly dependent on correct carboxylation. Warfarin decrease blood coagulation by inhibiting VKOR, an enzyme that recycles oxidized vitamin K to its reduced form after it has participated in the carboxylation of several blood coagulation proteins, mainly prothrombin, factor VII, IX and X. For this reason, drugs in this class are also referred to as vitamin K antagonists [28]. Common polymorphisms in the gene Vitamin K epoxide Reductase Complex subunit 1 (VKORC1) affect warfarin dose response and blood clotting through effects on the formation of the reduced form of vitamin K, which subsequently alters carboxylation of vitamin K-dependent hemostatic and nonhemostatic proteins.
Polymorphism in the VKORC1 gene explains 30% of the dosevariation between patients. A promoter polymorphism of VKORC1- 1639G>A is present in linkage disequilibrium with VKORC1 1173C>T. This polymorphism in the promoter region alters the binding site for VKORC1 transcription factor and leads to lower VKORC1 mRNA expression and protein in human liver. As a result, the steady-state concentration of tissue VKOR decreases making a person with this variation more susceptible to inhibition by warfarin [29]. Thus -1639G>A of VKORC1, is associated with an increased sensitivity to warfarin [30]. There are two main groups formed due to particular sequence of nucleotides called haplotypes - Low dose haplotype group (A) and High dose haplotype group (B). Adenine nucleotide of G-1639A (rs9923231) polymorphism and thymine of C1173T (rs8050894) polymorphism represents “A†haplotype while complementary wild type nucleotides represents “B†haplotype. Dose decreases from wild type to variant haplotype i.e. from B to A [31,32]. Thus, patients starting warfarin therapy who are - 1639A carriers require lower initial doses of the drug than - 1639G carriers. The - 1639G>A allele frequency varies among different ethnic groups. It is the major allele (around 90%) in Asian populations, and may be a contributing factor for lower warfarin dosing requirements often observed in patients of Asian descent. It is also common in Caucasians (around 40%) and African Americans (around 14%) [33,34].
Genetic testing for drug like Warfarin enables us to predictvariability in drug responsiveness. It has the potential to reduce trialand error as well as risks of therapy. There is general agreement amongpublished retrospective population studies that the combination of VKORC1 and CYP2C9 genotypes, together with gender, age, body mass index or height or weight and concurrent medications, predict approximately 50% of warfarin dose requirement [35].
Individuals with CYP2C9 *1/*1, VKORC1 - 1639GG genotype are known to require the highest amount of weekly dose and are sometimes known as extensive or ultra-metabolizers [36,37]. There is strong evidence that patients who are homozygous or heterozygous for CYP2C9*2, *3 or/and VKORC1 G - 1639A are sensitive to warfarin and acenocoumarol. They require low maintenance dose (CYP2C9*2, *3 carriers requiring reduction in dose by 16% and 41%for heterozygous and homozygous carriers, respectively) and take a longer time to achieve stable dose with international normalized ratio INR) values within the therapeutic range (2.0 to 3.0-3.5) [37].
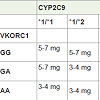
The FDA approved classification of genotype-guided warfarindose estimates have been derived from multiple published clinical studies and are as given below; with *1/*1, AA; *1/*3, GG; *1/*3, AG; *1/*2, AG; *1/*2, AA; *2/*2, GG; *2/*2, AG and *2/*3, GG are recommended 3.0 to 4.0 mg/day and those with genotypes *1/*1, GG; *1/*1, AG and *1/*2, GG have been suggested a higher dose range of 5.0 to 7.0 mg/day. The revised dosing recommendations also highlight the prolonged time to achieve therapeutic INR effect for a given dosage regimen in carriers of CYP2C9 *1/*3, *2/*2, *2/*3, and *3/*3 as compared to non-carriers of the above CYP2C9 genotypes (*1/*1; *1/*2). The guidelines, based on several published studies, also emphasize the increased risk of over anticoagulation (INR>4) or bleeding in CYP2C9 *2 and *3 carriers [38]. In 2007, reference to genetic factors while prescribing warfarin was included in USA by the FDA. Later in 2010, FDA included detailed dosing guidance for the various genotypes in the form of a table in the warfarin product inserts. Table 1 depicts the same. In this, ranges are derived from multiple published clinical studies. CYP2C9*2 and *3 as well as VKORC1 - 1639G>A (rs9923231) variant is used in this Table 2. Other co-inherited VKORC1 variants may also be important determinants of warfarin dose.
Various algorithms have been derived for simplifying the dosecalculation as per genotypes and clinical information. Sconce et al. had demonstrated the first algorithm as follows [38]:
Warfarin dose (mg/day) = [0.628 - 0.0135*(Age) - 0.24*(CYP2C9*2) - 0.37*(CYP2C9*3) - 0.241*(VKORC1) + 0.0162*(height)] 2
(Wild type, heterozygous and homozygous genotypes of CYP2C9*2 and *3 were coded as 0, 1 and 2 respectively, while that of VKORC1 were coded as 1, 2 and 3 respectively).
The risk of bleeding associated with warfarin therapy is greatest during the commencement of therapy when the optimum dose for the patient is being evaluated. The algorithms that are available for predicting stable therapeutic dose could be important in maximizing the benefit of using genotypes to guide anticoagulant dose at the beginning so that patient reaches and can remain in the target INR for longer period and experience less complications.
Frequencies of CYP2C9 and VKORC1 Polymorphisms inIndia
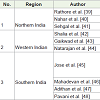
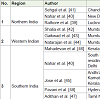
CYP2C9*2: The prevalence of CYP2C9*2 allele frequencies from different studies have been documented in Table 2. In North Indian population, Rathore et al. have reported the allelic frequency of CYP2C9*2 to be 0.049, which is similar to the frequencies reported by Nahar et al. (0.05) and Sehgal et al. (0.05) [39-41]. In study including subjects from Western region of India, Shalia et al. have counted the variant allele frequency of CYP2C9*2 as 0.046 which was similar to other studies by Gaikwad et al. and Natarajan et al. frequencies of 0.04 and 0.054 respectively [42-44]. In South Indianpopulation, CYP2C9*2 frequencies have been communicated by Jose et al. (0.04) and Mahadevan et al. (0.03) while Adithan et al. in TamilNadu, reported the frequency of CYP2C9*2 to be 0.026 [45-47]. Whereas, Pavani et al. in their study in the South region (Hyderabad) have reported the frequency of CYP2C9*2 allele as 0.085, which was higher as compared to the other regions [48]. In another study by Nahar et al. which included the South Indian residing in the Northern region, the frequency of CYP2C9*2 occurred to be 0.006 which was relatively lesser than other studies conducted in Southern India [40]. This discrepancy would be due to the smaller sample size of South Indian included in their study and may also be due to variation in the ethno-geographic origin of the subjects. Thus CYP2C9*2 allele frequency ranges from 0.05 to 0.026 from north to south of India exception being study by Pavani et al. of 0.085 [48]. The frequencies of CYP2C9*2 was found to be similar in other Asian countries (0.029) [24], but lower than the Caucasians (0.151) [36].
CYP2C9*3: Different studies demonstrating CYP2C9*3 allele frequencies have been listed in Table 3. In North Indian populations, Rathore et al. have counted frequency of CYP2C9*3 allele as 0.039, which was much lower than reported by the other two studies by Nahar et al. ( 0.11), and Sehgal et al. (0.17) [39-41]. In Western Region of India, Shalia et al. and Gaikwad et al. have stated CYP2C9*3 allele frequencies as 0.122 and 0.129 respectively near to that reported by Nahar et al. and Sehgal et al. [40-43]. Whereas, Natarajan et al. did not find CYP2C9*3 variant allele in their study population [44]. In South Indian population, Nahar et al., Jose et al., Pavani et al. and Adithan et al. have reported CYP2C9*3 allele frequency in decreasing order as 0.09, 0.08, 0.067, 0.065 respectively [40,45,47,48]. Contradicting to all above studies a recent study by Mahadevan et al. in the population of Kerala, have shown higher preponderance of CYP2C9*3 allele (0.15) [46]. Thus, overall CYP2C9*3 allele frequency observed were higher from North to South (0.065-0.17) than reported for Caucasians (0.057) [25,49-53]. Exceptions are by Rathore et al. who reported it 0.039 similar to other Asian populations and by Natarajan et al. who have reported it to be absent in western region [25,39,44].
VKORC1 G-1639A: VKORC1-1639A allele occurrence in various parts as stated by different studies are documented in Table 4. In the studies including North Indian population, Nahar et al. have reported VKORC1 -1639G>A minor allele occurring at the frequency of 0.19, slightly higher than Sehgal et al. (0.15) and Rathore et al. (0.142) [39,40]. In the Western region, Shalia et al. have stated prevalence of the minor allele of VKORC1 (-1639A) with frequency of 0.13, which is comparable to that reported by Gaikwad et al. as 0.127 and Natarajan et al. as 0.13. Nahar et al. conducted a study involving South Indians residing in Northern region, and have reported the frequency of VKORC1 (-1639A) to be 0.14, similar to that reported in Northern and Western region [40,42-44]. Pavani et al. have reported the VKORC1 (-1639A) frequency as 0.119, which was comparable to the above stated frequency [48]. In a recent study including South Indian population of Kerala by Mahadevan et al. the allelic frequency of VKORC1 (-1639 A) was reported to be 0.075 [46]. Thus, the VKORC1 (-1639A) ranged from 0.19 to 0.075 from North to South of India similar to that reported for Japanese [33] lower than reported for Caucasians [31,32,34] and higher than those of Africans [34].
Giri et al. analyzed the allele frequency distributions of CYP2C9*2, CYP2C9*3 and VKORC1 (-1639G>A) polymorphisms in population with origin from five different states; Punjab, Haryana, Delhi, Uttar Pradesh (UP) and Bihar in the northern part of India [49-54]. Table 5 summarizes the rare allelic frequencies of this INDICO study. The overall CYP2C9*3 and VKORC1 -1639A allele frequencies were comparable to other studies while CYP2C9*2 allele frequencies were greater than reported by others.
Conclusion
The present review highlights that prevalence of warfarinsensitive polymorphisms in Indian population, not only did the polymorphisms vary compared to other populations, but also showed regional variations from North to South. The prevalence of rare allele frequencies was highest for VKORC1 -1639A, followed by CYP2C9*3 and then CYP2C9*2. According to the literature, an individual, carrying the *3 allele of CYP2C9 gene is more important than carrying VKORC1 variant allele, since VKORC1 variant allele only contributes to 25-30% reduction in warfarin dosage as opposed to almost 95% reduction explained by CYP2C9*3 variant [33-35]. But on a population level, VKORC1 -1639A is more important simply because it is found in higher frequency in our population. These factors may be taken into consideration for genome based dosing regimen for oral anticoagulant therapies in future. The information on genetic factors that affect warfarin sensitivity has not been incorporated into daily practice at our end. Perhaps more practical approach for clinicians would be to take genotype information in to consideration along with other factors when dosing warfarin, tobegin with.
Acknowledgements
The authors would like to acknowledge Sir H.N. Hospital andResearch Centre for recruitment of patients and Sir H.N. MedicalResearch Society for financial support for the corresponding originalstudy carried out.
References
- Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, et al. (2004) Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost 91: 87-94.
- Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, et al. (2009) A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5: e1000433.
- Koutrouvelis A, Abouleish A, Indrikovs A, Alperin J (2010) Case scenario: emergency reversal of oral anticoagulation. Anesthesiology 113: 1192-1197.
- (2010) COUMADIN® TABLETS (Warfarin Sodium Tablets, USP) Crystalline COUMADIN® FOR INJECTION (Warfarin Sodium for Injection, USP) Antocoagulant. 25: 1-39.
- Wittkowsky AK (2003) Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. SeminVasc Med 3: 221-230.
- Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, et al. (2001) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119 (Suppl): 8S-21S.
- De Caterina R, Husted S, Wallentin L, Agnelli G, Bachmann F, et al. (2007) Anticoagulants in heart disease: current status and perspectives. Eur Heart J 28: 880-913.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons, Bonow RO, et al. (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of ThoracicSurgeons. Circulation: 114: e84-231.
- Van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briët E (1993) Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med 153: 1557-1562.
- Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, et al. (1995) Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med 333: 11-7.
- Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, et al. (1996) Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 348: 423-428.
- Eckman MH, Levine HJ, Pauker SG (1993) Effect of laboratory variation in the prothrombin-time ratio on the results of oral anticoagulant therapy. N Engl J Med 329: 696-702.
- British Committee for Standards in Haematology (1998) Guidelines on oral anticoagulation: Third edition. Br J Haematol 101: 374-387.
- Khan T, Wynne H, Wood P, Torrance A, Hankey C, et al. (2004) Dietary vitamin K influences intra-individual variability in anticoagulant response to warfarin. Br J Haematol 124: 348-354.
- Hirsh J, Fuster V, Ansell J, Halperin JL, American Heart Association/American College of Cardiology Foundation (2003) American Heart Association/ American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol 41: 1633-1652.
- Strubbins MJ, Harries LW, Smith G, Tarbit MH, Wolf CR (1996) Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics 6: 429-439.
- Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired S-Warfarin metabolism catalysed by R144C allelic variant of CYP2C9. Pharmacogenetics 4: 39-42.
- Haining RL, Hunter AP, Veronese ME, Trager WF, Rettie AE (1996) Allelic variants of human cytochrome P450 2C9: baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity, andprochiral selectivity of the wild-type and I359L mutant forms. Arch Biochem Biophys 333: 447-458.
- Crespi CL, Miller VP (1997) The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH: cytochrome P450 oxidoreductase. Pharmacogenetics 7: 203-210.
- Linder MW, Bon Homme M, Reynolds KK, Gage BF, Eby C, et al. (2009) Interactive modelling for ongoing utility of pharmacogenetic diagnostic testing: application for warfarin therapy. Clin Chem 55: 1861-1868.
- Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, et al. (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287: 1690- 1698.
- Aithal GP, Day CP, Kesteven PJ, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk ofbleeding complications. Lancet 353: 717-719.
- Lindh JD, Holm L Andersson ML Rane A (2009) Influence of CYP2C9 genotype on warfarin dose requirements--a systematic review and metaanalysis. Eur J Clin Pharmacol 65: 365-375.
- PharmGKB (2012) Haplotype CYP2C9*2. Stanford University.
- Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11: 781-791.
- PharmGKB (2012) Haplotype CYP2C9*3. Stanford University.
- Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, et al. (2011) Clinicalpharmacogenetics implementation consortium guidelines for CYP2C9 andVKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 90: 625-639.
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, et al. (2004) The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(3 Suppl): 204S-233S.
- Zhu Y, Shennan M, Reynolds KK, Johnson NA, Herrnberger MR, et al. (2007) Estimation of warfarin maintenance dose based on VKORC1 (-1639G>A) and CYP2C9 genotypes. Clin Chem 53: 1199-1205.
- PharmGKB (2012) Haplotype CYP2C9*2. Stanford University.
- D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, et al. (2005) A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 105: 645-649.
- Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, et al. (2005) A novel functional VKORC1 promoter polymorphism is associated with interindividual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14: 1745-1751.
- Obayashi K, Nakamura K, Kawana J, Ogata H, Hanada K, et al. (2006) VKORC1 gene variations are the major contributors of variation in warfarin dose in Japanese patients. Clin Pharmacol Ther 80: 169-178.
- Ross KA, Bigham AW, Edwards M, Gozdzik A, Suarez-Kurtz G, et al. (2010) Worldwide allele frequency distribution of four polymorphisms associated with warfarin dose requirements. J Hum Genet 55: 582-589.
- Daly AK (2009) Pharmacogenomics of anticoagulants: steps toward personal dosage. Genome Med 1: 10.
- Takahashi H ,Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, et al. (2006) Different contributions of polymorphisms in VKORC1 and CYP2C9 to intraand inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 16: 101-110.
- Aithal GP, Day CP, Kesteven PJ, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353: 717-719.
- Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, et al. (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106: 2329-2333.
- Rathore SS, Agarwal SK, Pande S, Mittal T, Mittal B (2010) Frequencies of VKORC1 -1639 G>A, CYP2C9*2 and CYP2C9*3 genetic variants in Northern Indian Population. Biosci Trends 4: 333-337.
- Nahar R, Deb R, Saxena R, Puri RD, Verma IC (2013) Variability in CYP2C9 allele frequency: a pilot study of its predicted impact on warfarin response among healthy South and North Indians. Pharmacol Rep 65: 187-194.
- Sehgal T, Hira JK, Ahluwalia J, Das R, Vijayvergiya R, et al. (2015) High prevalence of VKORC1*3 (G9041A) genetic polymorphism in north Indians: a study on patients with cardiac disorders on acenocoumarol. Drug Discov Ther 9: 404-410.
- Shalia KK, Doshi SM, Parikh S, Pawar PP, Divekar SS, et al. (2012) ORC1 and CYP2C9 gene polymorphisms in Indian population and its effect on warfarin response. J Assoc Physicians India 60: 34-38.
- Gaikwad T, Ghosh K, Kulkarni B, Kulkarni V, Ross C, et al. (2013) Influence of CYP2C9 and VKORC1 gene polymorphisms on warfarin dosage, over anticoagulation and other adverse outcomes in Indian population. Eur J Pharmacol 710: 80-84.
- Natarajan S, Ponde CK, Rajani RM, Jijina F, Gursahani R, et al. (2013) Effect of CYP2C9 and VKORC1 genetic variations on warfarin dose requirements in Indian patients. Pharmacol Rep 65: 1375-1382.
- Jose R, Chandrasekaran A, Sam SS, Gerard N, Chanolean S, et al. (2005) CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the south Indian population. Fundam Clin Pharmacol 19: 101-105.
- Mahadevan L, Yesudas A, Sajesh PK, Revu S, Kumar P, et al. (2014) Prevalence of genetic variants associated with cardiovascular disease risk and drug response in the Southern Indian population of Kerala. Indian J Hum Genet 20: 175-184
- Adithan C, Gerard N, Vasu S, Balakrishnan R, Shashindran CH, et al. (2003) Allele and genotype frequency of CYP2C9 in Tamilnadu population. Eur J ClinPharmacol 59: 707-709.
- Pavani A, Naushad SM, Rupasree Y, Kumar TR, Malempati AR, et al. (2012) Optimization of warfarin dose by population-specific pharmacogenomic algorithm. Pharmacogenomics J 12: 306-311.
- Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, et al. (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106: 135-140.
- Markatos CN, Grouzi E, Politou M, Gialeraki A, Merkouri E, et al. (2008) VKORC1 and CYP2C9 allelic variants influence acenocoumarol dose requirements in Greek patients. Pharmacogenomics 9: 1631-1638.
- Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, et al. (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121: 23-34.
- Yang JQ, Morin S, Verstuyft C, Fan LA, Zhang Y, et al. (2003) Frequency of cytochrome P450 2C9 allelic variants in the Chinese and French populations. Fundam Clin Pharmacol 17: 373-376.
- Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, et al. (1999) Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun 254: 628-631.
- Giri AK, Khan NM, Grover S, Kaur I, Basu A, et al. (2014) C9, CYP4F2 and VKORC1 genes associatedwith warfarin dosage inthe Indian population. Pharmacogenomics 15: 1337-54.