Research Article
Assortment of Cold-Active Alkaline Protease-Producing Bacterial Species Isolated From Three Different Lake Samples of Kashmir, India
Furhan J and Sharma S*
Department of Biotechnology and Microbiology, Arni University, India
Corresponding author: Sharma S, Department of Biotechnology and Microbiology, Arni School of Basic Sciences, Arni University, Himachal Pradesh-176401, India, Tel no: +91-9816535399; E-mail: sarikasharma19@gmail.com
Citation: Furhan J, Sharma S. Assortment of Cold-Active Alkaline Protease-Producing Bacterial Species Isolated From Three Different Lake Samples of Kashmir, India. J Environ Soc Sci. 2018;5(1): 136
Copyright ©2018 Furhan J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Environmental and Social Sciences | Volume: 5, Issue: 1
Submission: 03/02/2018; Accepted: 27/02/2018; Published: 03/03/2018
Abstract
Extensively used application was applied to achieve cold-active alkaline protease-producing microorganisms for Lake Samples from Kashmir, India. About 100 µl dilutions of soil sample were initially allowed for microbial growth by long term incubation at cold temperatures in nutrient agar medium. Appropriate colonies of bacteria were observed, marked and picked from nutrient agar plates and further screened at different temperatures (5-20 °C) and alkaline pH (6-12) conditions. Amongst 28 potential bacterial isolates, 5 protease producing isolates were optimized for cultural conditions and were found to be producing maximum amount of enzymes in between 15 to 25 °C and pH 9 to 10, classifying the isolates as psychrotrophic and alkalitolerants. The selected 5 isolates belonged to four different genera i.e. Staphylococcus, Serratia, Bacillus and Pseudomonas. The crude enzyme from Bacillus (WLCP1) was further characterized showing maximum protease activity at pH 10 and 15 °C respectively suggesting the biotechnological potential of the isolate.
Keywords:
Kashmir; Cold-active proteases; Psychrotrophic; Temperature; pH; Bacillus
Introduction
Microorganisms tailored to environments with low temperatures have been differentiated as psychrophiles and psychrotrophs [1], capable of producing cold-active enzymes such as proteases [2]. Proteases are found extensively within microbial population namely bacteria, actinomycetes, viruses and fungi. Amid the rising demand in industries and global market, researchers have successfully produced proteases from the various microbial sources [3]. Since the beginning of enzymology, microbial proteases have been studied widely and are considered as most important kind of hydrolytic enzymes. These enzymes have gained significant attention in the industrial sector mainly due their key role in cellular metabolic processes and proteolytic activity [4]. The proteases available today in the market are derived mainly from microbial sources which share two-third of commercial protease production in the world [5,6]. Recently, there has been an increased emphasis on cold active enzymes produced by microorganisms existing permanently in cold habitats located in polar zones at high altitudes on deep sea. Among cold active enzymes, cold active alkaline proteases generally constitute an important group of enzymes which are active at ambient temperature and having a high catalytic efficiency so that many of the industrial processes utilizing them could be made more cost effective by reducing the consumption of energy [7]. Furthermore, these protease producing bacteria working at low temperatures can play a vital role in treatment of household and industrial waste rich in proteins. Cold-active proteases have applications in textile, food and leather industry and can also be used as detergent additives for cold washing, cleansing of contact lens and much more [4,8]. Different ecosystems with low temperatures have been explored for protease producing cold-adapted microorganisms, such as glaciers [9,10], cold desert soil [11], alpines and sandy sediments [12,13]. But psychrophilic and psychrotrophic bacteria from lakes of Kashmir exposed to cold temperature haven’t been explored for cold-active enzymes. Keeping in view the biotechnological interest for protease producing cold adapted microbes, the present exploration was under taken to analyze the diversity of cold-active protease-producing bacteria in Lake Sediments of three different lakes of Kashmir, India.
Materials and Methods
Sampling
Soil samples were collected from lake sediments of three different lakes viz., Wular Lake (latitude 34° 19’ 60.00” N, longitude 74° 35’ 59.99” E), Manasbal Lake (latitude 34° 14’ 60.00” N, longitude 74° 39’ 59.99” E) and Dal Lake (latitude 34° 06’ 60.00” N, longitude 74° 51’ 59.99” E) of Kashmir, India in the month of December, when the air temperature is in between 3.4-8.2 °C.
Collection and pre-treatment of soil sediment samples
Samples from each lake were obtained at a depth of 4-5 cm where most of the microbial activity takes place. Soil sediment samples were collected by using sediment sampler, transferred in a well packed box sealed in ice to avoid the contamination and instantaneously transported to laboratory, Arni University and stored at -20 °C until further processing. The moisture was removed by air drying the soil samples initially and then passed through a 2 mm brass sieve to remove any debris and other undesirable things. About 1 g freshly sieved soil from each sample was taken for the isolation of microbes [14].
Isolation of cold-active strains from soil sample
Under sterile conditions, 1g of soil sample was taken and mixed it with 9 ml of sterile distilled cold water by swirling the tube upside down few times and subjecting the test tube for vortexing. Samples were serially diluted up to eight dilutions (10-8). About 100 µl aliquot of appropriate dilutions were added onto sterile petri dishes of nutrient agar medium at pH 7.4. Plates were incubated at varying cold conditions (5-20 °C) and observed daily for the growth potential bacterial colonies for 7 days. Morphologically dissimilar colonies were chosen and purified by repetitive streaking on the similar agar medium.
Screening of cold-active isolates for proteases activity
A loopful of purified bacterial cultures were grown in 250 ml flasks containing 50 ml of nutrient broth and appropriate broth culture was inoculated on skim milk agar plates (1.5% peptone, 1% agar, 0.5% sodium chloride, 1% skimmed milk and pH 7). The plates were incubated at varying cold conditions at 5 °C, 10 °C and 20 °C, and regularly checked for the formation of clear halo zone after 48-72 hr interval. The isolates showing clear halo zone indicated protease production and were further screened for enzyme production at wide range of temperatures (5-20 °C) and pH (6-12) on skimmed milk agar and casein agar medium.
Cultural conditions of protease producing isolates
To optimize cultural conditions of isolates, the optimum temperature for enzyme production of the microorganisms was determined by incubating the isolates in freshly prepared 5ml nutrient broth at different temperatures (5, 10, 15, 25 and 37 °C) and pH (7 to 12) for 48 h. The enzyme activity was determined in the supernatant and Optical Density (OD) was recorded at 660nm using spectrophotometer.
Morphological and biochemical characterization of coldactive protease producing strains
Based on physical and microscopic appearance, the strains were observed for colony size, colour, margin, arrangement, texture, spore formation and motility followed by Gram’s staining of bacterial colonies. Furthermore, series of tests were performed for identifying the bacterial strains up to genus level as outlined by Bergey’s Manual of Determinative Bacteriology and ABIS online [15].
Protease assay
The protease activity was determined with casein as a substrate according to the method described previously [16]. The reaction mixture contained 100mM of Tris-HCl (pH 8.5), 0.6 mg/ml of casein, and 0.5 ml crude enzyme solution. The mixture was incubated at 15 °C for 10 min and the reaction was stopped by the addition of an equal volume of 10% trichloroacetic acid. After centrifugation, the end products in the supernatant were determined by the Folin-Ciocalteus reagent and absorbance of supernatant was read at 600 nm. One unit of protease activity was defined as the amount of enzyme required to liberate l µg/ml tyrosine.
Characterization of crude alkophilic protease from WLCP1
Determination of optimum temperature: The effect of temperature on protease activity was assayed at different temperatures from 5 to 50 °C by adding equal amount of enzyme in casein (1%) substrate. The enzyme and substrate were preincubated separately under assaying conditions for 10 mins before mixing them together.
Determination of optimum pH: To check the effect of pH on the activity of enzyme, the enzyme assay at pH values ranging from 6 to 12 were performed using appropriate buffers: citrate buffer (6.0-7.0), phosphate buffer (pH 8), Sodium buffer (pH 9.0-10.0) and KCl/NaOH (pH 11.0-12.0) respectively.
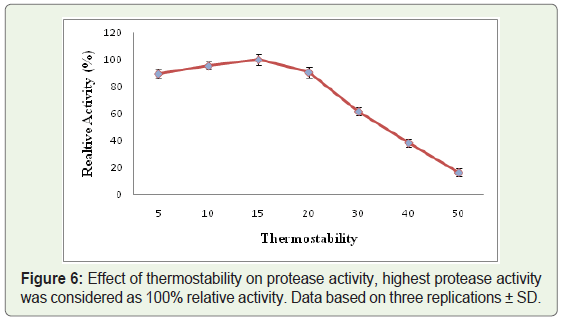
Thermostability: To find out thermal stability, the enzyme was preincubated for 1 h at different temperatures (5-50 °C), followed by determination of residual activity with added substrate (50µl autoclaved casein) at pH 10.
Statistical analysis: Statistical analysis was carried out using SPSS software. The experiments were conducted in triplicate and values are represented as mean with standard errors.
Results
Isolation, screening and optimization of isolates
A total number of 72 bacterial isolates were collectively isolated from three soil samples of three different lakes. Out of all the collected isolates, 28 bacterial strains were found to be positive for protease production. Based on further screening of these positive isolates for protease production, five isolates showing enzyme production at wide range of temperature and pH 9.0-11.0 was selected (Figure 1).
Among positive strains, 2 isolates were selected from both S-1 and S-2 Sample and 1 isolate was selected from S-3 (Table 1).
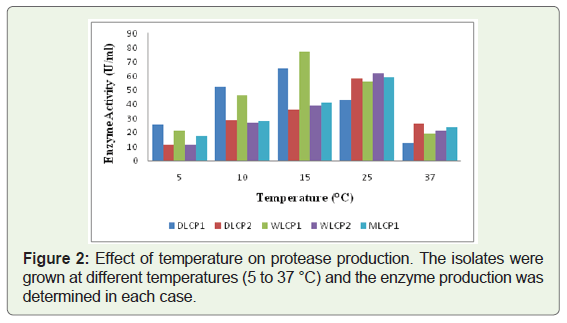
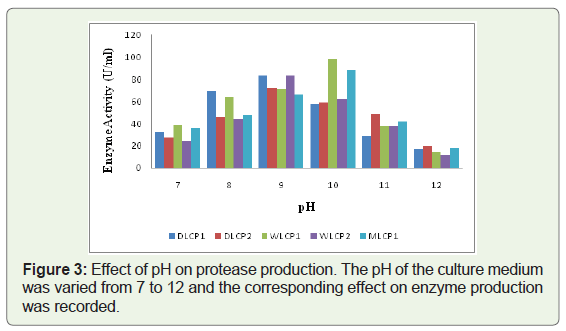
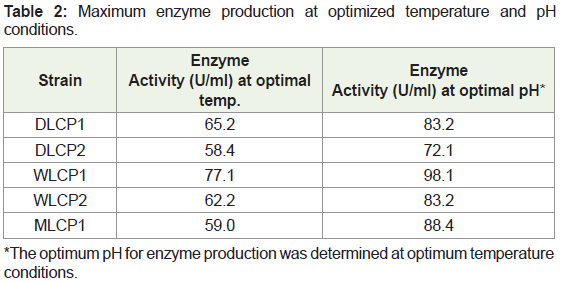
All five selected isolates were tested for their optimum growth temperature and were found to be psychrotrophic in nature with an optimal enzyme production between 15 and 25 °C. Two isolates (DLCP1, WLCP1) showed the lowest temperature for enzyme production at around 15 °C, whereas rest of the three isolates (DLCP2, WLCP2, and MLCP1) showed optimal enzyme production at around 25 °C. The maximum enzyme productions at optimal temperatures for DLCP1, DLCP2, WLCP1, WLCP2, and MLCP1 were found 64.2 U/ml, 58.4 U/ml, 77.1 U/ml, 66.2 U/ml, 59.0 U/ml, respectively (Figure 2). The optimal pH for enzyme production of isolates was in between 9.0 to 10.0. Three isolates (DLCP1, DLCP2, and WLCP2) showed maximum enzyme production at pH 9.0 whereas two isolates (WLCP1, MLCP1) showed maximum enzyme production at pH 10.0. The maximum enzyme productions at optimal pH for DLCP1, DLCP2, WLCP1, WLCP2, and MLCP1 were found 83.2 U/ml, 72.1 U/ml, 98.1 U/ml, 83.2 U/ml, 88.4 U/ml, respectively (Figure 3). The enzyme production of all the isolates was increased after growing them under these optimized conditions of temperature and pH (Table 2).
*The optimum pH for enzyme production was determined at optimum temperature conditions.
Morphological and biochemical characterization of positive isolates
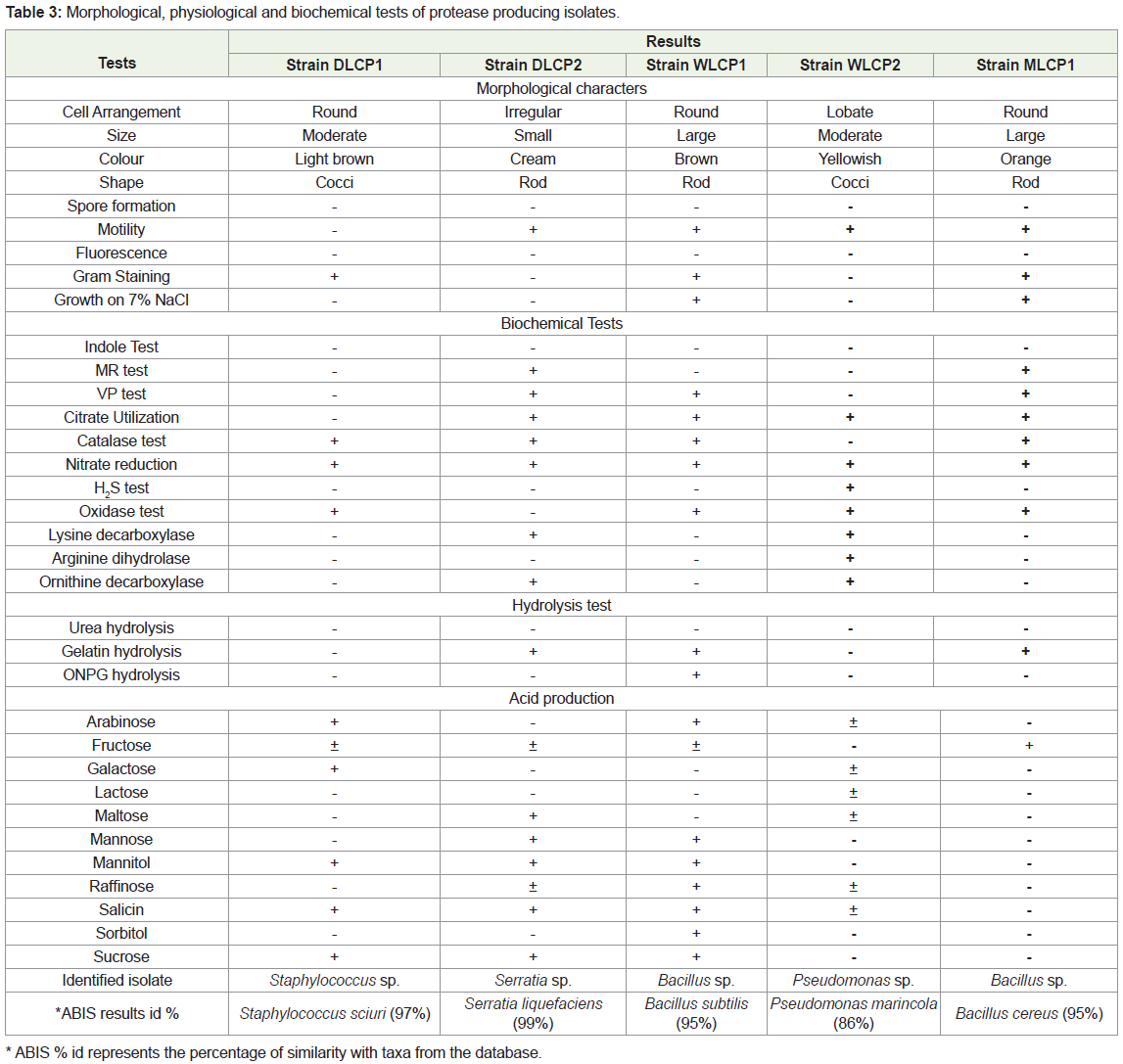
The bacterial isolates (DLCP1, DLCP2, WLCP1, WLCP2, MLCP1) from three different soil samples (S-1, S-2 and S-3) showing highest protease production were subjected to different morphological, physiological and biochemical tests for genus level identification. The results were compared with Bergey’s manual of determinative bacteriology as well as bacterial identification software ABIS online for accurate identification. Results showed that isolate DLCP1 belonged to genus Staphylococcus showing 97% similarity with taxa Staphylococcus sciuri. Isolate DLCP2 belonged to genus Serratia showing 99% similarity with taxa Serratia liquefaciens. Isolate WLCP1 and MLCP1 belonged to genus Bacillus and showed highest similarity (95%) with taxa Bacillus subtilis and Bacillus cereus respectively. The isolate WLCP2 belonged to genus Pseudomonas and showed 86% of similarity with taxa Pseudomonas marincola (Table 3).
Characterization of crude alkalophilic protease WLCP1
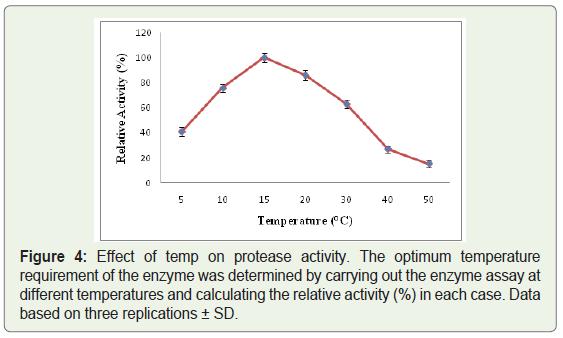
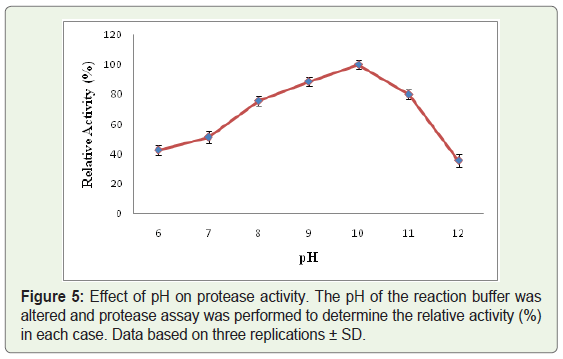
Out of all the protease producing isolates, one of the bacterial strains designated as WLCP1 was selected for further studies depending upon the largest zone of clearance at cold temperature, the crude protease was characterized for pH, temperature and thermostability by performing protease activity assay. The crude protease produced by strain WLCP1 showed optimal protease activity at 15 °C and lost only 12 % of activity at 20 °C; however the activity substantially declined at 30 °C and kept on declining further with increase in temperature. The maximum protease activity was considered as 100% relative activity (Figure 4). The crude enzyme was active in the wide range of pH and optimum protease activity was recorded at pH 10 and retained more than 50% of protease activity below and above the optimum pH (Figure 5). The enzyme was relatively stable between 5 to 30 °C at pH 10, there was a sharp decline in stability at 40 °C and enzyme was sensitive to temperature at 50 °C (Figure 6).
Discussion
Proteases are considered as the most important group of industrial enzymes accounting for more than half of the total sale of enzymes globally [5], and find applications in the food and feed, leather, detergent and pharmaceutical industries [3]. Keeping in view the biotechnological interest of proteases from cold-adapted microorganisms, we isolated psychrotrophic bacteria from three different lakes (Wular Lake, Manasbal Lake and Dal Lake) positioned in different regions of Kashmir, India. These lakes are exposed to cold temperatures during the winter season in the valley making the environment ideal for growth of psychrophiles and psychrotrophic organisms. To our knowledge, these lakes haven’t been explored for protease producing microorganisms. Therefore, it would be of much interest to explore their environment for production of imperative enzymes such as proteases secreted by cold adapted organisms. Previously Joshi, et al. reported psychrotrophic bacterium producing moderately halotolerant, SDS stable alkaline protease from Nainital Lake, located in Uttaranchal state, India [17]. In present study, the bacterial species isolated from three different lake sediment samples of Kashmir were screened on alkaline casein agar medium and skim milk agar medium. The method of isolating microbes by screening the cultures on above mentioned screening media has been used extensively in previous studies [18,19]. Isolates were grown under alkaline and cold conditions; the optimum temperature of selected isolates was in between 15 to 25 °C, classifying them as psychrotrophic which are known to produce various kinds of enzymes that can be vital in basic and industrial applications [20]. The isolates showed growth in pH range of 7 to 11, indicating the alkalophilic nature of isolates, and previous studies confirm that microorganisms able to withstand and grow in alkaline conditions produce enzymes that find applications in detergent industry and bioremediation [3]. Previous studies confirm that Bacillus sp. has been reported abundantly for alkaline protease production and also exploited at commercial level with high success rate [18,21-23]. The isolates in present study belonged to the different genera showing the diversity of cold environment strains presentwithin the lake sediment samples. The characterization of crude protease from strain WLCP1 showed maximum protease activity at 15 °C, classifying the protease as cold-active. The protease produced by psychrotrophic Bacillus cereus and Bacillus subtilis displayed maximum activity at 20 °C [17,24], but unlike the present case, their temperature optima is higher than the crude protease of strain WLCP1. However similar to our finding the protease isolated from Stenotrophomonas sp. showed maximal activity at 15 °C [25]. The optimum pH for the activity of the protease of strain WLCP1 was 10, indicating that the enzyme produced is alkaline protease. Mostly the pH of alkaline proteases reported from psychrotrophic bacteria falls in between 9-10 [17,24,25]. Previous reports strongly demonstrate the biotechnological importance of proteases that can grow and survive under cold and alkaline conditions [10,26,27].
The present study expands our knowledge of psychrotrophic bacteria present in famous yet unexplored lakes of Kashmir. Moreover these unexplored cold environments can serve as source of enzymes that show characteristics of industrial importance.
Acknowledgement
Authors are thankful to Arni University for providing the necessary research facility.
References
- Morita RJ (1975) Psychrophilic bacteria. Bacteriol Rev 39: 144-167.
- Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1: 200-208.
- Kumar CG, Takagi H (1999) Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 17: 561-594.
- Joshi S, Satyanarayana T (2013) Biotechnology of cold-active proteases. Biology 2: 755-783.
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62: 597-635.
- Beg QK, Gupta R (2003) Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb Technol 32: 294-304.
- Kuddus M, Ramteke PW (2012) Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol 38: 330-338.
- Kasana RC (2010) Proteases from psychrotrophs: an overview. Crit Rev Microbiol 36: 134-145.
- Kasana RC (2010) Proteases from psychrotrophs: an overview. Crit Rev Microbiol 36: 134-145.
- Kuddus M, Ramteke PW (2009) Cold-active extracellular alkaline protease from an alkaliphilic Stenotrophomonas maltophilia: production of enzyme and its industrial applications. Can J Microbiol 55: 1294-1301.
- Kasana RC, Yadav SK (2007) Isolation of a psychrotrophic Exiguobacterium sp. SKPB5 (MTCC 7803) and characterization of its alkaline protease. Curr Microbiol 54: 224-229.
- Margesin R, Schinner F (1991) Characterization of a metalloprotease from psychrophilic Xanthomonas maltophilia. FEMS Microbiol Lett 79: 257-261.
- Yu Y, Li HR, Zeng YX, Chen B (2011) Bacterial diversity and bioprospecting for cold-active hydrolytic enzymes from culturable bacteria associated with sediment from Nella Fjord, Eastern Antarctica. Mar Drugs 9: 184-195.
- Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, et al. (2002) Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem 277: 432-438.
- Bergey D, Holt JG (1994) Bergey’s manual of determinative bacteriology. The Williams Wilkins Co.
- Takami H, Akiba T, Horikoshi K (1989) Production of extremely thermostable alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biotechnol 30: 120-124.
- Joshi GK, Kumar S, Sharma V (2007) Production of moderately halotolerant, SDS stable alkaline protease from Bacillus cereus MTCC 6840 isolated from Lake Nainital, Uttaranchal state, India. Braz J Microbiol 38: 773-779.
- Shi JS, Wu QF, Xu ZH, Tao WY (2005) Identification of psychrotrophs SYP-A2-3 producing cold-adapted protease from the No. 1 Glacier of China and study on its fermentation conditions. Wei Sheng Wu Xue Bao 45: 258-263.
- Mothe T, Sultanpuram VR (2016) Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus. 3 Biotech 6: 53.
- Gomes J, Steiner W (2004) The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol 42: 223-235.
- Kim JI, Lee SM, Jung HJ (2005) Characterization of calcium-activated bifunctional peptidase of the psychrotrophic Bacillus cereus. J Microbiol 43: 237-243.
- Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63: 735-750.
- Khan F (2013) New microbial proteases in leather and detergent industries. Innovative Research in Chemistry 1: 1-6.
- Baghel VS, Tripathi RD, Ramteke PW, Gopal K, Dwivedi S, et al. (2005) Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India. Enzyme Microb Technol 36: 654-659.
- Saba I, Qazi PH, Rather SA, Dar RA, Qadri QA, et al. (2012) Purification and characterization of a cold active alkaline protease from Stenotrophomonas sp., isolated from Kashmir, India. World J Microbiol Biotechnol 28: 1071-1079.
- Dube S, Singh L, Alam S (2001) Proteolytic anaerobic bacteria from lake sediments of Antarctica. Enzyme Microb Technol 28: 114-121.
- Huston AL (2008) Biotechnological aspects of cold-adapted enzymes. In Psychrophiles: from biodiversity to biotechnology. Springer pp: 347-363.